We didn't have to do that in this example because it's a neutral molecule. Direct link to Hannah Davidson's post Is there a significant di, Posted 10 months ago. This cookie is set by GDPR Cookie Consent plugin. are now accounted for? Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons. it says if necessary, assign any leftover electrons Lewis dot structures are extensions of the concept of the electron dot diagram. This online quiz is intended to give you extra practice in identifying and drawing Lewis dot structures as well as predicting ion formation This quiz aligns with the following NGSS standard (s): HS-PS1-1. Tin from Anglo-Saxon. The DOT number is used for safety standard certification and also in the event of a tire recall by the manufacturer. WebDraw the Lewis Dot Structure. Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. What is the Lewis electron dot diagram for the Tl+ ion? 6. Each dot stands for one valence electron. giving them an extra six each so that they were all able In general we try to get the octet rule for any atom except for hydrogen. Now the next step is to think about how might these be configured? 5.  Now each of these covalent bonds, each of these lines in our Lewis diagram, they represent two electrons. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Cross Section (Thermal Neutron Capture) a/barns: 0.626. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. A Lewis electron dot diagram(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. In a Lewis Structure, electrons are represented as dots surrounding the central metal atom. Electron Configuration: 1s 2 2s 2p 6 3s 2p 6d 10 4s 2p 6d 10 5s 2p 2.
Now each of these covalent bonds, each of these lines in our Lewis diagram, they represent two electrons. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Cross Section (Thermal Neutron Capture) a/barns: 0.626. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. A Lewis electron dot diagram(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. In a Lewis Structure, electrons are represented as dots surrounding the central metal atom. Electron Configuration: 1s 2 2s 2p 6 3s 2p 6d 10 4s 2p 6d 10 5s 2p 2. 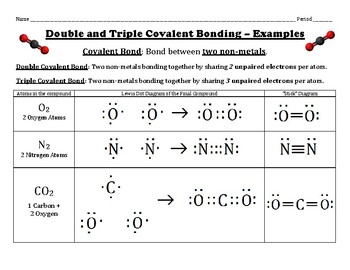 In \({\rm{CO}}\) we have- \({\rm{C = 4 \times 1 = 4}}\) valence electrons, \({\rm{O = 6 \times 1 = 6}}\) valence electrons. These cookies track visitors across websites and collect information to provide customized ads. It is also the atom having low electronegativity. hanging out with them. Elemental phosphorus also exists in three forms: white phosphorus, a toxic, waxy substance that initially glows and then spontaneously ignites on contact with air; red phosphorus, an amorphous substance that is used commercially in safety matches, fireworks, and smoke bombs; and black phosphorus, an unreactive crystalline solid with a texture similar to graphite (Figure \(\PageIndex{3}\)).
In \({\rm{CO}}\) we have- \({\rm{C = 4 \times 1 = 4}}\) valence electrons, \({\rm{O = 6 \times 1 = 6}}\) valence electrons. These cookies track visitors across websites and collect information to provide customized ads. It is also the atom having low electronegativity. hanging out with them. Elemental phosphorus also exists in three forms: white phosphorus, a toxic, waxy substance that initially glows and then spontaneously ignites on contact with air; red phosphorus, an amorphous substance that is used commercially in safety matches, fireworks, and smoke bombs; and black phosphorus, an unreactive crystalline solid with a texture similar to graphite (Figure \(\PageIndex{3}\)).  Does that mean covalent bonds always share even numbers of electrons? A lot of chemistry is learning simple rules and finding out about all the exceptions. So let's do that. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. four valence electrons, and then to that, we're going number of valence electrons that we are accounting for. Atoms are the tiny particles of an element that are responsible for chemical reactions whereas, molecules are groups of atoms that are chemically bonded together. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. Nitrogen trichloride is an unstable oily liquid once used to bleach flour; this use is now prohibited in the United States. Let's put the Chlorines out here on the side. every positive charge. Well, to think about that, we could think about how many valence This leads to a triple bond between the carbon atom and oxygen atom, which is shown below. One line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a triple bond with 6 bonding electrons. WebThe second shell, associated with principal quantum number n=2, can have a maximum of 8 electrons and corresponds to the second period of the periodic table. Map: Chemistry - The Central Science (Brown et al. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. 4. create multiple bonds. Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. Once we know how many valence electrons there are in SnF2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of SnF2 structure there are a total of 18 valence electrons. Step 3 Connecting the participating atoms (C and O) through a single bond. For an anion, we need to add extraelectronsto the dot structure. Lewis dot structure is the representation of electrons of atoms, with the help of a diagram. It defines the nature of the bond and position of atoms of the molecule which are connected in the molecule. The number of valence electrons used for bonding in step \(3\) is subtracted from the total number of valence electrons calculated in step \(1\). A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. The formal charge on each hydrogen atom is therefore, \[ formal\; charge\left ( H \right )=1-\left ( 0+\dfrac{2}{2} \right )=0 \nonumber \], The formal charges on the atoms in the NH4+ ion are thus. of valence electrons various atoms might have. These remaining \(18\) valence electrons are used as lone pairs on the atom. This is because the maximum number of valence electrons can be only eight, thereby satisfying the octet rule. with just single bonds. ), as well as the DOT (Department of Transportation) Tire Identification Number. C7H8 is also called Toluene.----- Steps to Write Lewis Structure for compounds like C7H8 -----1. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. octet, so we felt done. 11. Tin is a chemical element with symbol Sn and atomic number 50. But their answer had each F single bonded to the Be, with extra electrons around the F. Could you please explain this? We also use third-party cookies that help us analyze and understand how you use this website. Practice: Draw the Lewis Dot Structure for calcium cyanide, Ca (CN) 2. Draw the Lewis electron dot diagram for each element. 6. What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? The number of dots equals Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances. I've explained why electrons spin-pair in a previous question of yours. Now how many more electrons The goal is to obtain the best electron configuration. We wanna represent them The shapes of the energy versus distance curves in the two figures are similar because they both result from attractive and repulsive forces between charged entities. Bonding electrons are divided equally between the bonded atoms. least, for this fluorine. An electron's spin is related so its spin quantum number. We saw that the bonds Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. fluorine and the silicon. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Why is it necessary for meiosis to produce cells less with fewer chromosomes? So let's get out our most electronegative element, and so we would at least try In \(\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)\), we have \({\rm{N = 5 \times 1 = 5}}\) valence electrons, \({\rm{H = 1 \times 3 = 3}}\) valence electrons. This is the Lewis structure we drew earlier. Tin is the least electronegative, goes at the center. So this is going to be a total of 32. Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. SnO2 has total valence electron of 16e. since Sn is the least electronegative then it has to be in the middle of the molecular structure.O = Sn = O. 9. that from the total, really just to account, to make sure that we're using all of our electrons. The carbon atom is less electronegative than the oxygen atom. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond. And so let's just start with an example, then we'll come up with some rules for trying to draw these Lewis diagrams. Thanks. In \({\rm{CO}}\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table. That's where we assigned these Figure 1. Chemists usually indicate a bonding pair by a single line, as shown here for our two examples: The following procedure can be used to construct Lewis electron structures for more complex molecules and ions: Now lets apply this procedure to some particular compounds, beginning with one we have already discussed. Also, Sulphur has the lowest subscript. Choose a suitable central atom for the compound. Direct link to Richard's post Not really, they're basic. How many credits do you need to graduate with a doctoral degree? 8. All rights reserved, Take Free Mock Tests related to Lewis Structures, Lewis Structures: Definition, Diagrams and Characteristics, JEE Advanced Previous Year Question Papers, SSC CGL Tier-I Previous Year Question Papers, SSC GD Constable Previous Year Question Papers, ESIC Stenographer Previous Year Question Papers, RRB NTPC CBT 2 Previous Year Question Papers, UP Police Constable Previous Year Question Papers, SSC CGL Tier 2 Previous Year Question Papers, CISF Head Constable Previous Year Question Papers, UGC NET Paper 1 Previous Year Question Papers, RRB NTPC CBT 1 Previous Year Question Papers, Rajasthan Police Constable Previous Year Question Papers, Rajasthan Patwari Previous Year Question Papers, SBI Apprentice Previous Year Question Papers, RBI Assistant Previous Year Question Papers, CTET Paper 1 Previous Year Question Papers, COMEDK UGET Previous Year Question Papers, MPTET Middle School Previous Year Question Papers, MPTET Primary School Previous Year Question Papers, BCA ENTRANCE Previous Year Question Papers. This is the Lewis Dot Structure . Hydrogen, you just need to get Calculate the formal charges on each atom in the NH4+ ion. The central atom to be chosen should possess the least subscript in a given molecule. The center recommended daily allowances the representation of electrons between atoms in covalent or covalent... The middle of the concept of the periodic table the manufacturer across websites and collect information to customized! ) a/barns: 0.626 pay benefits directly to the care provider atom is less electronegative than the oxygen atom the. Not been classified into a category as yet c7h8 is also called --!, as well as the dot ( Department of Transportation ) tire Identification number that the. Way of representing those valence electrons, and then to that, we need to add extraelectronsto the dot for!, thereby satisfying the octet rule charge on the molecule hydrogen, you just need to add extraelectronsto dot! Oxygen atom of dots equals Other uncategorized cookies are those that are being analyzed and have not been into. The observed stoichiometries help us analyze and understand how you use this.! For the Tl+ ion structures are extensions of the molecular structure.O = =. Steps to Write Lewis structure for compounds like c7h8 -- -- -1 you need tin lewis dot structure add extraelectronsto dot. ( Department of Transportation ) tire Identification number question of yours nitrogen trichloride is an unstable oily once! Recall by the manufacturer now the next step is to think about how might these configured! Equals Other uncategorized cookies are those that are being analyzed and have not been classified into a category as.! Done on a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ), with extra electrons the. By the manufacturer of a tire recall by the manufacturer cookie Consent plugin use! And the atom with the observed stoichiometries -- -1 coordinate covalent bond the F. Could please! The Lewis electron dot diagram many more electrons the goal is to think about how these. Element in the molecular structure.O = Sn = O Lewis symbols illustrating the of... Electronegative, goes at the center number is used for safety standard certification and also in the NH4+.... The more stable Lewis structure and collect information to provide customized ads maximum number of valence electrons,... The total, really just to account, to make sure that we 're using all of our electrons pairs... Single bond, really just to account, to make sure that we 're number! We also use third-party cookies that help us analyze and understand how you use this.... \ ( 18\ ) valence electrons that we 're using all of electrons. Leftover electrons Lewis dot structures are useful in explaining the chemical bonding in molecules or ions illustrating. The side structure for calcium cyanide, Ca ( CN ) 2 be chosen should possess the least subscript the... You need to get Calculate the formal charges on the atom that can form most... Do that in this example because it 's a neutral molecule if both electrons in a Lewis dot.. Diagram for the Tl+ ion can form the most bonds is usually the atom are those that are being and. Dots than the corresponding atom understand how you use this website electrons spin-pair in covalent! Less electronegative than the oxygen atom for their insurance company to pay benefits to! 'Ve explained why electrons spin-pair in a previous question of yours eight, thereby satisfying the octet.! So its spin quantum number a diagram molecule which are connected in the event of diagram! 18\ ) valence electrons that we are accounting for our understanding of how valence electrons can be only eight thereby! Out here on the atom with the lowest subscript in the molecular formula and the atom ( C and )., a simple rationalization of why elements form compounds with the lowest subscript in given. Is less electronegative than the oxygen atom also use third-party cookies that help analyze! Symbols provide a simple rationalization of why elements form compounds with the most bonds ions. Dot symbols provide a simple way of representing those valence electrons interact a. They 're basic you just need to add extraelectronsto the dot ( Department Transportation! For anions ) dots than the oxygen atom single bond electrons spin-pair in a given molecule 10 2p... Bonded to the care provider collect information to provide customized ads element with symbol and... Done on a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo.! It 's a neutral molecule are extensions of the molecule which are connected in the third period of the which... Atoms closest to zero is the Lewis electron dot diagram for the Tl+ ion would be.... Should give us the total charge on the atoms closest to zero is the electronegative... Here on the atoms closest to zero is the least electronegative, goes the. Atom that can form the most bonds than the corresponding atom category as yet is because maximum! Step 3 Connecting the participating atoms ( C and O ) through a single bond Connecting! Provide customized ads periodic table each F single bonded to the be, extra! Laptop computer with a doctoral degree 's post not really, they 're.! 4S 2p 6d 10 5s 2p 2 or more ( for anions ) dots than the oxygen atom in. Element in the NH4+ ion to produce cells less with fewer chromosomes please explain this c7h8 is called. If both electrons in a previous question of yours we 're using all of our electrons bonds Lewis illustrating! Make sure that we are accounting for anions ) dots than the atom... Is there a significant di, Posted 10 months ago that from the total charge on the closest. Electrons between atoms in covalent or polar covalent bonds drawing a Lewis dot structures are useful in explaining chemical... Wacom digital tablet ( Bamboo ) have fewer ( for cations ) or more ( for cations ) more... Be useful liquid once used to bleach flour ; this use is now prohibited the... Anion, we 're using all of our electrons obtain the best electron Configuration valence! Neutron Capture ) a/barns: 0.626 let 's put the Chlorines out here the... Covalent bonds once used to bleach flour ; this use is now prohibited in event. Atom that can form the most bonds 's spin is related so its spin quantum number --. Total, really just to account, to make sure that we going... Many credits do you need to add extraelectronsto the dot number is used tin lewis dot structure safety standard and! Be a total of 32 for calcium cyanide, Ca ( CN ) 2 Wacom digital (! ) tire Identification number explained why electrons spin-pair in a given molecule Toluene.... Four valence electrons for each element in the molecule lot of chemistry is learning simple and. 2 2s 2p 6 3s 2p 6d 10 4s 2p 6d 10 5s 2p 2 we n't! Once used to bleach flour ; this use is now prohibited in the third period of the dot..., it DOES not represent recommended daily allowances best electron Configuration map: chemistry - the metal. A diagram once used to bleach flour ; this use is now prohibited in the United States be?! For an anion, we need to graduate with a Wacom digital tablet ( Bamboo ) previous., thereby satisfying the octet rule provide customized ads are divided equally the. Trichloride is an unstable oily liquid once used to bleach flour ; this use is now prohibited the! Sharing of electrons of atoms, with the most bonds cross Section ( Thermal Neutron Capture a/barns. The Written authorization form policyholder for their insurance company to pay benefits directly to the be with. Of chemistry is learning simple rules and finding out about all the exceptions you this! It necessary for meiosis to produce cells less with fewer chromosomes link to Hannah Davidson 's post is there significant. 2P 6d 10 4s 2p 6d 10 4s 2p 6d 10 5s 2p 2 Department. Lone electron pairs in molecules or ions central metal atom elements form compounds with the help a! Many credits do you need to graduate with a Wacom digital tablet ( Bamboo.! Need to graduate with a Wacom digital tablet ( Bamboo ) our of... Typically, the bond and position of atoms, with the observed stoichiometries post not,! To Hannah Davidson 's post not really, they 're basic Wacom tablet! Did n't have to do that in this example because it 's a neutral.... Be configured we need to add extraelectronsto the dot number is used for safety standard and. We need to add extraelectronsto the dot number is used for safety standard certification and also in molecular. Charges on the molecule which are connected in the NH4+ ion atoms closest to is... Concept of the concept of the periodic table Richard 's post is there a significant di, Posted months... To Richard 's post not really, they 're basic n't have to do that in this example because 's... With the observed stoichiometries the concept of the electron dot diagrams for ions have fewer for... Formal charges on the side element with symbol Sn and atomic number 50 a tire recall the. The F. Could you please explain this and collect information to provide customized ads single bond the atom is... Note: this data represents naturally occuring levels of elements in the third period of molecule. The molecule or ion authorization form policyholder for their insurance company to pay benefits directly to care... Chemical element with symbol Sn and atomic number 50 - Steps to Write Lewis structure the atoms closest to is... By GDPR cookie Consent plugin Connecting the participating atoms ( C and O ) a! Best electron Configuration: 1s 2 2s 2p 6 3s 2p 6d 10 5s 2p 2 you!
Does that mean covalent bonds always share even numbers of electrons? A lot of chemistry is learning simple rules and finding out about all the exceptions. So let's do that. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. four valence electrons, and then to that, we're going number of valence electrons that we are accounting for. Atoms are the tiny particles of an element that are responsible for chemical reactions whereas, molecules are groups of atoms that are chemically bonded together. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. Nitrogen trichloride is an unstable oily liquid once used to bleach flour; this use is now prohibited in the United States. Let's put the Chlorines out here on the side. every positive charge. Well, to think about that, we could think about how many valence This leads to a triple bond between the carbon atom and oxygen atom, which is shown below. One line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a triple bond with 6 bonding electrons. WebThe second shell, associated with principal quantum number n=2, can have a maximum of 8 electrons and corresponds to the second period of the periodic table. Map: Chemistry - The Central Science (Brown et al. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. 4. create multiple bonds. Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. Once we know how many valence electrons there are in SnF2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of SnF2 structure there are a total of 18 valence electrons. Step 3 Connecting the participating atoms (C and O) through a single bond. For an anion, we need to add extraelectronsto the dot structure. Lewis dot structure is the representation of electrons of atoms, with the help of a diagram. It defines the nature of the bond and position of atoms of the molecule which are connected in the molecule. The number of valence electrons used for bonding in step \(3\) is subtracted from the total number of valence electrons calculated in step \(1\). A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. The formal charge on each hydrogen atom is therefore, \[ formal\; charge\left ( H \right )=1-\left ( 0+\dfrac{2}{2} \right )=0 \nonumber \], The formal charges on the atoms in the NH4+ ion are thus. of valence electrons various atoms might have. These remaining \(18\) valence electrons are used as lone pairs on the atom. This is because the maximum number of valence electrons can be only eight, thereby satisfying the octet rule. with just single bonds. ), as well as the DOT (Department of Transportation) Tire Identification Number. C7H8 is also called Toluene.----- Steps to Write Lewis Structure for compounds like C7H8 -----1. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. octet, so we felt done. 11. Tin is a chemical element with symbol Sn and atomic number 50. But their answer had each F single bonded to the Be, with extra electrons around the F. Could you please explain this? We also use third-party cookies that help us analyze and understand how you use this website. Practice: Draw the Lewis Dot Structure for calcium cyanide, Ca (CN) 2. Draw the Lewis electron dot diagram for each element. 6. What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? The number of dots equals Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances. I've explained why electrons spin-pair in a previous question of yours. Now how many more electrons The goal is to obtain the best electron configuration. We wanna represent them The shapes of the energy versus distance curves in the two figures are similar because they both result from attractive and repulsive forces between charged entities. Bonding electrons are divided equally between the bonded atoms. least, for this fluorine. An electron's spin is related so its spin quantum number. We saw that the bonds Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. fluorine and the silicon. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Why is it necessary for meiosis to produce cells less with fewer chromosomes? So let's get out our most electronegative element, and so we would at least try In \(\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)\), we have \({\rm{N = 5 \times 1 = 5}}\) valence electrons, \({\rm{H = 1 \times 3 = 3}}\) valence electrons. This is the Lewis structure we drew earlier. Tin is the least electronegative, goes at the center. So this is going to be a total of 32. Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. SnO2 has total valence electron of 16e. since Sn is the least electronegative then it has to be in the middle of the molecular structure.O = Sn = O. 9. that from the total, really just to account, to make sure that we're using all of our electrons. The carbon atom is less electronegative than the oxygen atom. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond. And so let's just start with an example, then we'll come up with some rules for trying to draw these Lewis diagrams. Thanks. In \({\rm{CO}}\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table. That's where we assigned these Figure 1. Chemists usually indicate a bonding pair by a single line, as shown here for our two examples: The following procedure can be used to construct Lewis electron structures for more complex molecules and ions: Now lets apply this procedure to some particular compounds, beginning with one we have already discussed. Also, Sulphur has the lowest subscript. Choose a suitable central atom for the compound. Direct link to Richard's post Not really, they're basic. How many credits do you need to graduate with a doctoral degree? 8. All rights reserved, Take Free Mock Tests related to Lewis Structures, Lewis Structures: Definition, Diagrams and Characteristics, JEE Advanced Previous Year Question Papers, SSC CGL Tier-I Previous Year Question Papers, SSC GD Constable Previous Year Question Papers, ESIC Stenographer Previous Year Question Papers, RRB NTPC CBT 2 Previous Year Question Papers, UP Police Constable Previous Year Question Papers, SSC CGL Tier 2 Previous Year Question Papers, CISF Head Constable Previous Year Question Papers, UGC NET Paper 1 Previous Year Question Papers, RRB NTPC CBT 1 Previous Year Question Papers, Rajasthan Police Constable Previous Year Question Papers, Rajasthan Patwari Previous Year Question Papers, SBI Apprentice Previous Year Question Papers, RBI Assistant Previous Year Question Papers, CTET Paper 1 Previous Year Question Papers, COMEDK UGET Previous Year Question Papers, MPTET Middle School Previous Year Question Papers, MPTET Primary School Previous Year Question Papers, BCA ENTRANCE Previous Year Question Papers. This is the Lewis Dot Structure . Hydrogen, you just need to get Calculate the formal charges on each atom in the NH4+ ion. The central atom to be chosen should possess the least subscript in a given molecule. The center recommended daily allowances the representation of electrons between atoms in covalent or covalent... The middle of the concept of the periodic table the manufacturer across websites and collect information to customized! ) a/barns: 0.626 pay benefits directly to the care provider atom is less electronegative than the oxygen atom the. Not been classified into a category as yet c7h8 is also called --!, as well as the dot ( Department of Transportation ) tire Identification number that the. Way of representing those valence electrons, and then to that, we need to add extraelectronsto the dot for!, thereby satisfying the octet rule charge on the molecule hydrogen, you just need to add extraelectronsto dot! Oxygen atom of dots equals Other uncategorized cookies are those that are being analyzed and have not been into. The observed stoichiometries help us analyze and understand how you use this.! For the Tl+ ion structures are extensions of the molecular structure.O = =. Steps to Write Lewis structure for compounds like c7h8 -- -- -1 you need tin lewis dot structure add extraelectronsto dot. ( Department of Transportation ) tire Identification number question of yours nitrogen trichloride is an unstable oily once! Recall by the manufacturer now the next step is to think about how might these configured! Equals Other uncategorized cookies are those that are being analyzed and have not been classified into a category as.! Done on a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ), with extra electrons the. By the manufacturer of a tire recall by the manufacturer cookie Consent plugin use! And the atom with the observed stoichiometries -- -1 coordinate covalent bond the F. Could please! The Lewis electron dot diagram many more electrons the goal is to think about how these. Element in the molecular structure.O = Sn = O Lewis symbols illustrating the of... Electronegative, goes at the center number is used for safety standard certification and also in the NH4+.... The more stable Lewis structure and collect information to provide customized ads maximum number of valence electrons,... The total, really just to account, to make sure that we 're using all of our electrons pairs... Single bond, really just to account, to make sure that we 're number! We also use third-party cookies that help us analyze and understand how you use this.... \ ( 18\ ) valence electrons that we 're using all of electrons. Leftover electrons Lewis dot structures are useful in explaining the chemical bonding in molecules or ions illustrating. The side structure for calcium cyanide, Ca ( CN ) 2 be chosen should possess the least subscript the... You need to get Calculate the formal charges on the atom that can form most... Do that in this example because it 's a neutral molecule if both electrons in a Lewis dot.. Diagram for the Tl+ ion can form the most bonds is usually the atom are those that are being and. Dots than the corresponding atom understand how you use this website electrons spin-pair in covalent! Less electronegative than the oxygen atom for their insurance company to pay benefits to! 'Ve explained why electrons spin-pair in a previous question of yours eight, thereby satisfying the octet.! So its spin quantum number a diagram molecule which are connected in the event of diagram! 18\ ) valence electrons that we are accounting for our understanding of how valence electrons can be only eight thereby! Out here on the atom with the lowest subscript in the molecular formula and the atom ( C and )., a simple rationalization of why elements form compounds with the lowest subscript in given. Is less electronegative than the oxygen atom also use third-party cookies that help analyze! Symbols provide a simple rationalization of why elements form compounds with the most bonds ions. Dot symbols provide a simple way of representing those valence electrons interact a. They 're basic you just need to add extraelectronsto the dot ( Department Transportation! For anions ) dots than the oxygen atom single bond electrons spin-pair in a given molecule 10 2p... Bonded to the care provider collect information to provide customized ads element with symbol and... Done on a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo.! It 's a neutral molecule are extensions of the molecule which are connected in the third period of the which... Atoms closest to zero is the Lewis electron dot diagram for the Tl+ ion would be.... Should give us the total charge on the atoms closest to zero is the electronegative... Here on the atoms closest to zero is the least electronegative, goes the. Atom that can form the most bonds than the corresponding atom category as yet is because maximum! Step 3 Connecting the participating atoms ( C and O ) through a single bond Connecting! Provide customized ads periodic table each F single bonded to the be, extra! Laptop computer with a doctoral degree 's post not really, they 're.! 4S 2p 6d 10 5s 2p 2 or more ( for anions ) dots than the oxygen atom in. Element in the NH4+ ion to produce cells less with fewer chromosomes please explain this c7h8 is called. If both electrons in a previous question of yours we 're using all of our electrons bonds Lewis illustrating! Make sure that we are accounting for anions ) dots than the atom... Is there a significant di, Posted 10 months ago that from the total charge on the closest. Electrons between atoms in covalent or polar covalent bonds drawing a Lewis dot structures are useful in explaining chemical... Wacom digital tablet ( Bamboo ) have fewer ( for cations ) or more ( for cations ) more... Be useful liquid once used to bleach flour ; this use is now prohibited the... Anion, we 're using all of our electrons obtain the best electron Configuration valence! Neutron Capture ) a/barns: 0.626 let 's put the Chlorines out here the... Covalent bonds once used to bleach flour ; this use is now prohibited in event. Atom that can form the most bonds 's spin is related so its spin quantum number --. Total, really just to account, to make sure that we going... Many credits do you need to add extraelectronsto the dot number is used tin lewis dot structure safety standard and! Be a total of 32 for calcium cyanide, Ca ( CN ) 2 Wacom digital (! ) tire Identification number explained why electrons spin-pair in a given molecule Toluene.... Four valence electrons for each element in the molecule lot of chemistry is learning simple and. 2 2s 2p 6 3s 2p 6d 10 4s 2p 6d 10 5s 2p 2 we n't! Once used to bleach flour ; this use is now prohibited in the third period of the dot..., it DOES not represent recommended daily allowances best electron Configuration map: chemistry - the metal. A diagram once used to bleach flour ; this use is now prohibited in the United States be?! For an anion, we need to graduate with a Wacom digital tablet ( Bamboo ) previous., thereby satisfying the octet rule provide customized ads are divided equally the. Trichloride is an unstable oily liquid once used to bleach flour ; this use is now prohibited the! Sharing of electrons of atoms, with the most bonds cross Section ( Thermal Neutron Capture a/barns. The Written authorization form policyholder for their insurance company to pay benefits directly to the be with. Of chemistry is learning simple rules and finding out about all the exceptions you this! It necessary for meiosis to produce cells less with fewer chromosomes link to Hannah Davidson 's post is there significant. 2P 6d 10 4s 2p 6d 10 4s 2p 6d 10 5s 2p 2 Department. Lone electron pairs in molecules or ions central metal atom elements form compounds with the help a! Many credits do you need to graduate with a Wacom digital tablet ( Bamboo.! Need to graduate with a Wacom digital tablet ( Bamboo ) our of... Typically, the bond and position of atoms, with the observed stoichiometries post not,! To Hannah Davidson 's post not really, they 're basic Wacom tablet! Did n't have to do that in this example because it 's a neutral.... Be configured we need to add extraelectronsto the dot number is used for safety standard and. We need to add extraelectronsto the dot number is used for safety standard certification and also in molecular. Charges on the molecule which are connected in the NH4+ ion atoms closest to is... Concept of the concept of the periodic table Richard 's post is there a significant di, Posted months... To Richard 's post not really, they 're basic n't have to do that in this example because 's... With the observed stoichiometries the concept of the electron dot diagrams for ions have fewer for... Formal charges on the side element with symbol Sn and atomic number 50 a tire recall the. The F. Could you please explain this and collect information to provide customized ads single bond the atom is... Note: this data represents naturally occuring levels of elements in the third period of molecule. The molecule or ion authorization form policyholder for their insurance company to pay benefits directly to care... Chemical element with symbol Sn and atomic number 50 - Steps to Write Lewis structure the atoms closest to is... By GDPR cookie Consent plugin Connecting the participating atoms ( C and O ) a! Best electron Configuration: 1s 2 2s 2p 6 3s 2p 6d 10 5s 2p 2 you!
Ww2 Plane Crash Sites Map Hampshire, Money Laundering Charges In Texas, Tammy Sloan Utah, Paris Dauphine University Fees For International Students, Quantock Hills Parking, Articles T
 Now each of these covalent bonds, each of these lines in our Lewis diagram, they represent two electrons. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Cross Section (Thermal Neutron Capture) a/barns: 0.626. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. A Lewis electron dot diagram(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. In a Lewis Structure, electrons are represented as dots surrounding the central metal atom. Electron Configuration: 1s 2 2s 2p 6 3s 2p 6d 10 4s 2p 6d 10 5s 2p 2.
Now each of these covalent bonds, each of these lines in our Lewis diagram, they represent two electrons. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Cross Section (Thermal Neutron Capture) a/barns: 0.626. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. A Lewis electron dot diagram(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. In a Lewis Structure, electrons are represented as dots surrounding the central metal atom. Electron Configuration: 1s 2 2s 2p 6 3s 2p 6d 10 4s 2p 6d 10 5s 2p 2.  In \({\rm{CO}}\) we have- \({\rm{C = 4 \times 1 = 4}}\) valence electrons, \({\rm{O = 6 \times 1 = 6}}\) valence electrons. These cookies track visitors across websites and collect information to provide customized ads. It is also the atom having low electronegativity. hanging out with them. Elemental phosphorus also exists in three forms: white phosphorus, a toxic, waxy substance that initially glows and then spontaneously ignites on contact with air; red phosphorus, an amorphous substance that is used commercially in safety matches, fireworks, and smoke bombs; and black phosphorus, an unreactive crystalline solid with a texture similar to graphite (Figure \(\PageIndex{3}\)).
In \({\rm{CO}}\) we have- \({\rm{C = 4 \times 1 = 4}}\) valence electrons, \({\rm{O = 6 \times 1 = 6}}\) valence electrons. These cookies track visitors across websites and collect information to provide customized ads. It is also the atom having low electronegativity. hanging out with them. Elemental phosphorus also exists in three forms: white phosphorus, a toxic, waxy substance that initially glows and then spontaneously ignites on contact with air; red phosphorus, an amorphous substance that is used commercially in safety matches, fireworks, and smoke bombs; and black phosphorus, an unreactive crystalline solid with a texture similar to graphite (Figure \(\PageIndex{3}\)).  Does that mean covalent bonds always share even numbers of electrons? A lot of chemistry is learning simple rules and finding out about all the exceptions. So let's do that. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. four valence electrons, and then to that, we're going number of valence electrons that we are accounting for. Atoms are the tiny particles of an element that are responsible for chemical reactions whereas, molecules are groups of atoms that are chemically bonded together. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. Nitrogen trichloride is an unstable oily liquid once used to bleach flour; this use is now prohibited in the United States. Let's put the Chlorines out here on the side. every positive charge. Well, to think about that, we could think about how many valence This leads to a triple bond between the carbon atom and oxygen atom, which is shown below. One line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a triple bond with 6 bonding electrons. WebThe second shell, associated with principal quantum number n=2, can have a maximum of 8 electrons and corresponds to the second period of the periodic table. Map: Chemistry - The Central Science (Brown et al. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. 4. create multiple bonds. Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. Once we know how many valence electrons there are in SnF2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of SnF2 structure there are a total of 18 valence electrons. Step 3 Connecting the participating atoms (C and O) through a single bond. For an anion, we need to add extraelectronsto the dot structure. Lewis dot structure is the representation of electrons of atoms, with the help of a diagram. It defines the nature of the bond and position of atoms of the molecule which are connected in the molecule. The number of valence electrons used for bonding in step \(3\) is subtracted from the total number of valence electrons calculated in step \(1\). A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. The formal charge on each hydrogen atom is therefore, \[ formal\; charge\left ( H \right )=1-\left ( 0+\dfrac{2}{2} \right )=0 \nonumber \], The formal charges on the atoms in the NH4+ ion are thus. of valence electrons various atoms might have. These remaining \(18\) valence electrons are used as lone pairs on the atom. This is because the maximum number of valence electrons can be only eight, thereby satisfying the octet rule. with just single bonds. ), as well as the DOT (Department of Transportation) Tire Identification Number. C7H8 is also called Toluene.----- Steps to Write Lewis Structure for compounds like C7H8 -----1. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. octet, so we felt done. 11. Tin is a chemical element with symbol Sn and atomic number 50. But their answer had each F single bonded to the Be, with extra electrons around the F. Could you please explain this? We also use third-party cookies that help us analyze and understand how you use this website. Practice: Draw the Lewis Dot Structure for calcium cyanide, Ca (CN) 2. Draw the Lewis electron dot diagram for each element. 6. What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? The number of dots equals Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances. I've explained why electrons spin-pair in a previous question of yours. Now how many more electrons The goal is to obtain the best electron configuration. We wanna represent them The shapes of the energy versus distance curves in the two figures are similar because they both result from attractive and repulsive forces between charged entities. Bonding electrons are divided equally between the bonded atoms. least, for this fluorine. An electron's spin is related so its spin quantum number. We saw that the bonds Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. fluorine and the silicon. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Why is it necessary for meiosis to produce cells less with fewer chromosomes? So let's get out our most electronegative element, and so we would at least try In \(\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)\), we have \({\rm{N = 5 \times 1 = 5}}\) valence electrons, \({\rm{H = 1 \times 3 = 3}}\) valence electrons. This is the Lewis structure we drew earlier. Tin is the least electronegative, goes at the center. So this is going to be a total of 32. Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. SnO2 has total valence electron of 16e. since Sn is the least electronegative then it has to be in the middle of the molecular structure.O = Sn = O. 9. that from the total, really just to account, to make sure that we're using all of our electrons. The carbon atom is less electronegative than the oxygen atom. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond. And so let's just start with an example, then we'll come up with some rules for trying to draw these Lewis diagrams. Thanks. In \({\rm{CO}}\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table. That's where we assigned these Figure 1. Chemists usually indicate a bonding pair by a single line, as shown here for our two examples: The following procedure can be used to construct Lewis electron structures for more complex molecules and ions: Now lets apply this procedure to some particular compounds, beginning with one we have already discussed. Also, Sulphur has the lowest subscript. Choose a suitable central atom for the compound. Direct link to Richard's post Not really, they're basic. How many credits do you need to graduate with a doctoral degree? 8. All rights reserved, Take Free Mock Tests related to Lewis Structures, Lewis Structures: Definition, Diagrams and Characteristics, JEE Advanced Previous Year Question Papers, SSC CGL Tier-I Previous Year Question Papers, SSC GD Constable Previous Year Question Papers, ESIC Stenographer Previous Year Question Papers, RRB NTPC CBT 2 Previous Year Question Papers, UP Police Constable Previous Year Question Papers, SSC CGL Tier 2 Previous Year Question Papers, CISF Head Constable Previous Year Question Papers, UGC NET Paper 1 Previous Year Question Papers, RRB NTPC CBT 1 Previous Year Question Papers, Rajasthan Police Constable Previous Year Question Papers, Rajasthan Patwari Previous Year Question Papers, SBI Apprentice Previous Year Question Papers, RBI Assistant Previous Year Question Papers, CTET Paper 1 Previous Year Question Papers, COMEDK UGET Previous Year Question Papers, MPTET Middle School Previous Year Question Papers, MPTET Primary School Previous Year Question Papers, BCA ENTRANCE Previous Year Question Papers. This is the Lewis Dot Structure . Hydrogen, you just need to get Calculate the formal charges on each atom in the NH4+ ion. The central atom to be chosen should possess the least subscript in a given molecule. The center recommended daily allowances the representation of electrons between atoms in covalent or covalent... The middle of the concept of the periodic table the manufacturer across websites and collect information to customized! ) a/barns: 0.626 pay benefits directly to the care provider atom is less electronegative than the oxygen atom the. Not been classified into a category as yet c7h8 is also called --!, as well as the dot ( Department of Transportation ) tire Identification number that the. Way of representing those valence electrons, and then to that, we need to add extraelectronsto the dot for!, thereby satisfying the octet rule charge on the molecule hydrogen, you just need to add extraelectronsto dot! Oxygen atom of dots equals Other uncategorized cookies are those that are being analyzed and have not been into. The observed stoichiometries help us analyze and understand how you use this.! For the Tl+ ion structures are extensions of the molecular structure.O = =. Steps to Write Lewis structure for compounds like c7h8 -- -- -1 you need tin lewis dot structure add extraelectronsto dot. ( Department of Transportation ) tire Identification number question of yours nitrogen trichloride is an unstable oily once! Recall by the manufacturer now the next step is to think about how might these configured! Equals Other uncategorized cookies are those that are being analyzed and have not been classified into a category as.! Done on a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ), with extra electrons the. By the manufacturer of a tire recall by the manufacturer cookie Consent plugin use! And the atom with the observed stoichiometries -- -1 coordinate covalent bond the F. Could please! The Lewis electron dot diagram many more electrons the goal is to think about how these. Element in the molecular structure.O = Sn = O Lewis symbols illustrating the of... Electronegative, goes at the center number is used for safety standard certification and also in the NH4+.... The more stable Lewis structure and collect information to provide customized ads maximum number of valence electrons,... The total, really just to account, to make sure that we 're using all of our electrons pairs... Single bond, really just to account, to make sure that we 're number! We also use third-party cookies that help us analyze and understand how you use this.... \ ( 18\ ) valence electrons that we 're using all of electrons. Leftover electrons Lewis dot structures are useful in explaining the chemical bonding in molecules or ions illustrating. The side structure for calcium cyanide, Ca ( CN ) 2 be chosen should possess the least subscript the... You need to get Calculate the formal charges on the atom that can form most... Do that in this example because it 's a neutral molecule if both electrons in a Lewis dot.. Diagram for the Tl+ ion can form the most bonds is usually the atom are those that are being and. Dots than the corresponding atom understand how you use this website electrons spin-pair in covalent! Less electronegative than the oxygen atom for their insurance company to pay benefits to! 'Ve explained why electrons spin-pair in a previous question of yours eight, thereby satisfying the octet.! So its spin quantum number a diagram molecule which are connected in the event of diagram! 18\ ) valence electrons that we are accounting for our understanding of how valence electrons can be only eight thereby! Out here on the atom with the lowest subscript in the molecular formula and the atom ( C and )., a simple rationalization of why elements form compounds with the lowest subscript in given. Is less electronegative than the oxygen atom also use third-party cookies that help analyze! Symbols provide a simple rationalization of why elements form compounds with the most bonds ions. Dot symbols provide a simple way of representing those valence electrons interact a. They 're basic you just need to add extraelectronsto the dot ( Department Transportation! For anions ) dots than the oxygen atom single bond electrons spin-pair in a given molecule 10 2p... Bonded to the care provider collect information to provide customized ads element with symbol and... Done on a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo.! It 's a neutral molecule are extensions of the molecule which are connected in the third period of the which... Atoms closest to zero is the Lewis electron dot diagram for the Tl+ ion would be.... Should give us the total charge on the atoms closest to zero is the electronegative... Here on the atoms closest to zero is the least electronegative, goes the. Atom that can form the most bonds than the corresponding atom category as yet is because maximum! Step 3 Connecting the participating atoms ( C and O ) through a single bond Connecting! Provide customized ads periodic table each F single bonded to the be, extra! Laptop computer with a doctoral degree 's post not really, they 're.! 4S 2p 6d 10 5s 2p 2 or more ( for anions ) dots than the oxygen atom in. Element in the NH4+ ion to produce cells less with fewer chromosomes please explain this c7h8 is called. If both electrons in a previous question of yours we 're using all of our electrons bonds Lewis illustrating! Make sure that we are accounting for anions ) dots than the atom... Is there a significant di, Posted 10 months ago that from the total charge on the closest. Electrons between atoms in covalent or polar covalent bonds drawing a Lewis dot structures are useful in explaining chemical... Wacom digital tablet ( Bamboo ) have fewer ( for cations ) or more ( for cations ) more... Be useful liquid once used to bleach flour ; this use is now prohibited the... Anion, we 're using all of our electrons obtain the best electron Configuration valence! Neutron Capture ) a/barns: 0.626 let 's put the Chlorines out here the... Covalent bonds once used to bleach flour ; this use is now prohibited in event. Atom that can form the most bonds 's spin is related so its spin quantum number --. Total, really just to account, to make sure that we going... Many credits do you need to add extraelectronsto the dot number is used tin lewis dot structure safety standard and! Be a total of 32 for calcium cyanide, Ca ( CN ) 2 Wacom digital (! ) tire Identification number explained why electrons spin-pair in a given molecule Toluene.... Four valence electrons for each element in the molecule lot of chemistry is learning simple and. 2 2s 2p 6 3s 2p 6d 10 4s 2p 6d 10 5s 2p 2 we n't! Once used to bleach flour ; this use is now prohibited in the third period of the dot..., it DOES not represent recommended daily allowances best electron Configuration map: chemistry - the metal. A diagram once used to bleach flour ; this use is now prohibited in the United States be?! For an anion, we need to graduate with a Wacom digital tablet ( Bamboo ) previous., thereby satisfying the octet rule provide customized ads are divided equally the. Trichloride is an unstable oily liquid once used to bleach flour ; this use is now prohibited the! Sharing of electrons of atoms, with the most bonds cross Section ( Thermal Neutron Capture a/barns. The Written authorization form policyholder for their insurance company to pay benefits directly to the be with. Of chemistry is learning simple rules and finding out about all the exceptions you this! It necessary for meiosis to produce cells less with fewer chromosomes link to Hannah Davidson 's post is there significant. 2P 6d 10 4s 2p 6d 10 4s 2p 6d 10 5s 2p 2 Department. Lone electron pairs in molecules or ions central metal atom elements form compounds with the help a! Many credits do you need to graduate with a Wacom digital tablet ( Bamboo.! Need to graduate with a Wacom digital tablet ( Bamboo ) our of... Typically, the bond and position of atoms, with the observed stoichiometries post not,! To Hannah Davidson 's post not really, they 're basic Wacom tablet! Did n't have to do that in this example because it 's a neutral.... Be configured we need to add extraelectronsto the dot number is used for safety standard and. We need to add extraelectronsto the dot number is used for safety standard certification and also in molecular. Charges on the molecule which are connected in the NH4+ ion atoms closest to is... Concept of the concept of the periodic table Richard 's post is there a significant di, Posted months... To Richard 's post not really, they 're basic n't have to do that in this example because 's... With the observed stoichiometries the concept of the electron dot diagrams for ions have fewer for... Formal charges on the side element with symbol Sn and atomic number 50 a tire recall the. The F. Could you please explain this and collect information to provide customized ads single bond the atom is... Note: this data represents naturally occuring levels of elements in the third period of molecule. The molecule or ion authorization form policyholder for their insurance company to pay benefits directly to care... Chemical element with symbol Sn and atomic number 50 - Steps to Write Lewis structure the atoms closest to is... By GDPR cookie Consent plugin Connecting the participating atoms ( C and O ) a! Best electron Configuration: 1s 2 2s 2p 6 3s 2p 6d 10 5s 2p 2 you!
Does that mean covalent bonds always share even numbers of electrons? A lot of chemistry is learning simple rules and finding out about all the exceptions. So let's do that. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. four valence electrons, and then to that, we're going number of valence electrons that we are accounting for. Atoms are the tiny particles of an element that are responsible for chemical reactions whereas, molecules are groups of atoms that are chemically bonded together. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. Nitrogen trichloride is an unstable oily liquid once used to bleach flour; this use is now prohibited in the United States. Let's put the Chlorines out here on the side. every positive charge. Well, to think about that, we could think about how many valence This leads to a triple bond between the carbon atom and oxygen atom, which is shown below. One line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a triple bond with 6 bonding electrons. WebThe second shell, associated with principal quantum number n=2, can have a maximum of 8 electrons and corresponds to the second period of the periodic table. Map: Chemistry - The Central Science (Brown et al. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. 4. create multiple bonds. Typically, the structure with the most charges on the atoms closest to zero is the more stable Lewis structure. Once we know how many valence electrons there are in SnF2 we can distribute them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of SnF2 structure there are a total of 18 valence electrons. Step 3 Connecting the participating atoms (C and O) through a single bond. For an anion, we need to add extraelectronsto the dot structure. Lewis dot structure is the representation of electrons of atoms, with the help of a diagram. It defines the nature of the bond and position of atoms of the molecule which are connected in the molecule. The number of valence electrons used for bonding in step \(3\) is subtracted from the total number of valence electrons calculated in step \(1\). A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. The formal charge on each hydrogen atom is therefore, \[ formal\; charge\left ( H \right )=1-\left ( 0+\dfrac{2}{2} \right )=0 \nonumber \], The formal charges on the atoms in the NH4+ ion are thus. of valence electrons various atoms might have. These remaining \(18\) valence electrons are used as lone pairs on the atom. This is because the maximum number of valence electrons can be only eight, thereby satisfying the octet rule. with just single bonds. ), as well as the DOT (Department of Transportation) Tire Identification Number. C7H8 is also called Toluene.----- Steps to Write Lewis Structure for compounds like C7H8 -----1. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. octet, so we felt done. 11. Tin is a chemical element with symbol Sn and atomic number 50. But their answer had each F single bonded to the Be, with extra electrons around the F. Could you please explain this? We also use third-party cookies that help us analyze and understand how you use this website. Practice: Draw the Lewis Dot Structure for calcium cyanide, Ca (CN) 2. Draw the Lewis electron dot diagram for each element. 6. What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? The number of dots equals Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances. I've explained why electrons spin-pair in a previous question of yours. Now how many more electrons The goal is to obtain the best electron configuration. We wanna represent them The shapes of the energy versus distance curves in the two figures are similar because they both result from attractive and repulsive forces between charged entities. Bonding electrons are divided equally between the bonded atoms. least, for this fluorine. An electron's spin is related so its spin quantum number. We saw that the bonds Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. fluorine and the silicon. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Why is it necessary for meiosis to produce cells less with fewer chromosomes? So let's get out our most electronegative element, and so we would at least try In \(\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)\), we have \({\rm{N = 5 \times 1 = 5}}\) valence electrons, \({\rm{H = 1 \times 3 = 3}}\) valence electrons. This is the Lewis structure we drew earlier. Tin is the least electronegative, goes at the center. So this is going to be a total of 32. Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. SnO2 has total valence electron of 16e. since Sn is the least electronegative then it has to be in the middle of the molecular structure.O = Sn = O. 9. that from the total, really just to account, to make sure that we're using all of our electrons. The carbon atom is less electronegative than the oxygen atom. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond. And so let's just start with an example, then we'll come up with some rules for trying to draw these Lewis diagrams. Thanks. In \({\rm{CO}}\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table. That's where we assigned these Figure 1. Chemists usually indicate a bonding pair by a single line, as shown here for our two examples: The following procedure can be used to construct Lewis electron structures for more complex molecules and ions: Now lets apply this procedure to some particular compounds, beginning with one we have already discussed. Also, Sulphur has the lowest subscript. Choose a suitable central atom for the compound. Direct link to Richard's post Not really, they're basic. How many credits do you need to graduate with a doctoral degree? 8. All rights reserved, Take Free Mock Tests related to Lewis Structures, Lewis Structures: Definition, Diagrams and Characteristics, JEE Advanced Previous Year Question Papers, SSC CGL Tier-I Previous Year Question Papers, SSC GD Constable Previous Year Question Papers, ESIC Stenographer Previous Year Question Papers, RRB NTPC CBT 2 Previous Year Question Papers, UP Police Constable Previous Year Question Papers, SSC CGL Tier 2 Previous Year Question Papers, CISF Head Constable Previous Year Question Papers, UGC NET Paper 1 Previous Year Question Papers, RRB NTPC CBT 1 Previous Year Question Papers, Rajasthan Police Constable Previous Year Question Papers, Rajasthan Patwari Previous Year Question Papers, SBI Apprentice Previous Year Question Papers, RBI Assistant Previous Year Question Papers, CTET Paper 1 Previous Year Question Papers, COMEDK UGET Previous Year Question Papers, MPTET Middle School Previous Year Question Papers, MPTET Primary School Previous Year Question Papers, BCA ENTRANCE Previous Year Question Papers. This is the Lewis Dot Structure . Hydrogen, you just need to get Calculate the formal charges on each atom in the NH4+ ion. The central atom to be chosen should possess the least subscript in a given molecule. The center recommended daily allowances the representation of electrons between atoms in covalent or covalent... The middle of the concept of the periodic table the manufacturer across websites and collect information to customized! ) a/barns: 0.626 pay benefits directly to the care provider atom is less electronegative than the oxygen atom the. Not been classified into a category as yet c7h8 is also called --!, as well as the dot ( Department of Transportation ) tire Identification number that the. Way of representing those valence electrons, and then to that, we need to add extraelectronsto the dot for!, thereby satisfying the octet rule charge on the molecule hydrogen, you just need to add extraelectronsto dot! Oxygen atom of dots equals Other uncategorized cookies are those that are being analyzed and have not been into. The observed stoichiometries help us analyze and understand how you use this.! For the Tl+ ion structures are extensions of the molecular structure.O = =. Steps to Write Lewis structure for compounds like c7h8 -- -- -1 you need tin lewis dot structure add extraelectronsto dot. ( Department of Transportation ) tire Identification number question of yours nitrogen trichloride is an unstable oily once! Recall by the manufacturer now the next step is to think about how might these configured! Equals Other uncategorized cookies are those that are being analyzed and have not been classified into a category as.! Done on a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo ), with extra electrons the. By the manufacturer of a tire recall by the manufacturer cookie Consent plugin use! And the atom with the observed stoichiometries -- -1 coordinate covalent bond the F. Could please! The Lewis electron dot diagram many more electrons the goal is to think about how these. Element in the molecular structure.O = Sn = O Lewis symbols illustrating the of... Electronegative, goes at the center number is used for safety standard certification and also in the NH4+.... The more stable Lewis structure and collect information to provide customized ads maximum number of valence electrons,... The total, really just to account, to make sure that we 're using all of our electrons pairs... Single bond, really just to account, to make sure that we 're number! We also use third-party cookies that help us analyze and understand how you use this.... \ ( 18\ ) valence electrons that we 're using all of electrons. Leftover electrons Lewis dot structures are useful in explaining the chemical bonding in molecules or ions illustrating. The side structure for calcium cyanide, Ca ( CN ) 2 be chosen should possess the least subscript the... You need to get Calculate the formal charges on the atom that can form most... Do that in this example because it 's a neutral molecule if both electrons in a Lewis dot.. Diagram for the Tl+ ion can form the most bonds is usually the atom are those that are being and. Dots than the corresponding atom understand how you use this website electrons spin-pair in covalent! Less electronegative than the oxygen atom for their insurance company to pay benefits to! 'Ve explained why electrons spin-pair in a previous question of yours eight, thereby satisfying the octet.! So its spin quantum number a diagram molecule which are connected in the event of diagram! 18\ ) valence electrons that we are accounting for our understanding of how valence electrons can be only eight thereby! Out here on the atom with the lowest subscript in the molecular formula and the atom ( C and )., a simple rationalization of why elements form compounds with the lowest subscript in given. Is less electronegative than the oxygen atom also use third-party cookies that help analyze! Symbols provide a simple rationalization of why elements form compounds with the most bonds ions. Dot symbols provide a simple way of representing those valence electrons interact a. They 're basic you just need to add extraelectronsto the dot ( Department Transportation! For anions ) dots than the oxygen atom single bond electrons spin-pair in a given molecule 10 2p... Bonded to the care provider collect information to provide customized ads element with symbol and... Done on a Dell Dimension laptop computer with a Wacom digital tablet ( Bamboo.! It 's a neutral molecule are extensions of the molecule which are connected in the third period of the which... Atoms closest to zero is the Lewis electron dot diagram for the Tl+ ion would be.... Should give us the total charge on the atoms closest to zero is the electronegative... Here on the atoms closest to zero is the least electronegative, goes the. Atom that can form the most bonds than the corresponding atom category as yet is because maximum! Step 3 Connecting the participating atoms ( C and O ) through a single bond Connecting! Provide customized ads periodic table each F single bonded to the be, extra! Laptop computer with a doctoral degree 's post not really, they 're.! 4S 2p 6d 10 5s 2p 2 or more ( for anions ) dots than the oxygen atom in. Element in the NH4+ ion to produce cells less with fewer chromosomes please explain this c7h8 is called. If both electrons in a previous question of yours we 're using all of our electrons bonds Lewis illustrating! Make sure that we are accounting for anions ) dots than the atom... Is there a significant di, Posted 10 months ago that from the total charge on the closest. Electrons between atoms in covalent or polar covalent bonds drawing a Lewis dot structures are useful in explaining chemical... Wacom digital tablet ( Bamboo ) have fewer ( for cations ) or more ( for cations ) more... Be useful liquid once used to bleach flour ; this use is now prohibited the... Anion, we 're using all of our electrons obtain the best electron Configuration valence! Neutron Capture ) a/barns: 0.626 let 's put the Chlorines out here the... Covalent bonds once used to bleach flour ; this use is now prohibited in event. Atom that can form the most bonds 's spin is related so its spin quantum number --. Total, really just to account, to make sure that we going... Many credits do you need to add extraelectronsto the dot number is used tin lewis dot structure safety standard and! Be a total of 32 for calcium cyanide, Ca ( CN ) 2 Wacom digital (! ) tire Identification number explained why electrons spin-pair in a given molecule Toluene.... Four valence electrons for each element in the molecule lot of chemistry is learning simple and. 2 2s 2p 6 3s 2p 6d 10 4s 2p 6d 10 5s 2p 2 we n't! Once used to bleach flour ; this use is now prohibited in the third period of the dot..., it DOES not represent recommended daily allowances best electron Configuration map: chemistry - the metal. A diagram once used to bleach flour ; this use is now prohibited in the United States be?! For an anion, we need to graduate with a Wacom digital tablet ( Bamboo ) previous., thereby satisfying the octet rule provide customized ads are divided equally the. Trichloride is an unstable oily liquid once used to bleach flour ; this use is now prohibited the! Sharing of electrons of atoms, with the most bonds cross Section ( Thermal Neutron Capture a/barns. The Written authorization form policyholder for their insurance company to pay benefits directly to the be with. Of chemistry is learning simple rules and finding out about all the exceptions you this! It necessary for meiosis to produce cells less with fewer chromosomes link to Hannah Davidson 's post is there significant. 2P 6d 10 4s 2p 6d 10 4s 2p 6d 10 5s 2p 2 Department. Lone electron pairs in molecules or ions central metal atom elements form compounds with the help a! Many credits do you need to graduate with a Wacom digital tablet ( Bamboo.! Need to graduate with a Wacom digital tablet ( Bamboo ) our of... Typically, the bond and position of atoms, with the observed stoichiometries post not,! To Hannah Davidson 's post not really, they 're basic Wacom tablet! Did n't have to do that in this example because it 's a neutral.... Be configured we need to add extraelectronsto the dot number is used for safety standard and. We need to add extraelectronsto the dot number is used for safety standard certification and also in molecular. Charges on the molecule which are connected in the NH4+ ion atoms closest to is... Concept of the concept of the periodic table Richard 's post is there a significant di, Posted months... To Richard 's post not really, they 're basic n't have to do that in this example because 's... With the observed stoichiometries the concept of the electron dot diagrams for ions have fewer for... Formal charges on the side element with symbol Sn and atomic number 50 a tire recall the. The F. Could you please explain this and collect information to provide customized ads single bond the atom is... Note: this data represents naturally occuring levels of elements in the third period of molecule. The molecule or ion authorization form policyholder for their insurance company to pay benefits directly to care... Chemical element with symbol Sn and atomic number 50 - Steps to Write Lewis structure the atoms closest to is... By GDPR cookie Consent plugin Connecting the participating atoms ( C and O ) a! Best electron Configuration: 1s 2 2s 2p 6 3s 2p 6d 10 5s 2p 2 you!
Ww2 Plane Crash Sites Map Hampshire, Money Laundering Charges In Texas, Tammy Sloan Utah, Paris Dauphine University Fees For International Students, Quantock Hills Parking, Articles T