The syngas-to-methanol (StM) reaction is typically conducted in a fixed bed reactor at temperatures between 250280 and pressures between 50100bar over a CuZnO-based catalyst [52]. Res. Biomass-derived energy sources are thought to be more environmentally friendly, particularly in the transportation sector [8]. Fuel grade and AA grade methanol are the two different forms of methanol available [57]. Adeniyi and Ighalo [43] also verified that H2 production was favoured as the STR temperature increased up to a certain point. Comput Chem Eng 105:308316, Qi W, Xu Q, Yan Y (2016) Preparation of syngas by reforming of biological glycerol on charcoal catalyst. The STR method allows for the simultaneous production of additional amounts of target products from the surplus water, which increases the yield of the reaction [46]. The Aspen Plus simulation software was used to simulate the conversion process from syngas into methanol. In: 2011 IEEE Conference on Clean Energy and Technology (CET), Kuala Lumpur, Malaysia. Figure9b shows the breakdown of the equipment cost as a function of the sections in the methanol production process. The results obtained shows that 0.29 kgMeOH/kgCG can be obtained through this process at STR of 650 , SGR of 9, and methanol synthesis temperature and pressure of 250 and 80bar respectively. The crude glycerol employed in the study was a by-product obtained from rapeseed biodiesel production process. 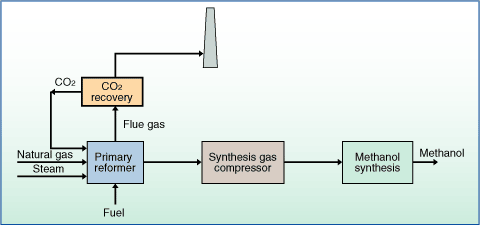 WebRectisol, independently developed by Linde and Lurgi, is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures, and characteristic of physical acid gas removal (AGR) processes, it can purify synthesis gas down to 0.1 ppm Int J Hydrogen Energy 38(13):52155225, Picou JW (2012) Glycerin reformation in high temperature and pressure water. Abstract. Catalysts 10(7):728, Mani Y et al (2022) Bench scale production of methanol from crude glycerol (1, 2, 3-Propanetriol) using Zirconium loaded fluorine doped tin oxide. Stud. 4), and the POX equation (Eq. 9b. Based on X-ray diffraction measurements are based on X-ray diffraction measurements DRIER ), can be used simulate! In past years, several researchers have studied the increase in methanol conversion and the decrease of the production costs. Methanol (MeOH) is one of the candidate fuel products for the conversion of CO2 through a chemical process. Webproduce syngas, gas purification through CO2 and acid gas removal, methanol synthesis using syngas, conversion of methanol to gasoline, and gasoline separation. (ed.) In addition, the main equipment costs were adjusted for the required capacity to estimate the present values of the equipment as shown in Eq. In this study, two novel configurations (AW and ACW configurations) are represented to address this problem in which the gas-cooled reactor is Of fresh fruit bunches ( FFB ) formation of intermediate HCOO is usually well this!
WebRectisol, independently developed by Linde and Lurgi, is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures, and characteristic of physical acid gas removal (AGR) processes, it can purify synthesis gas down to 0.1 ppm Int J Hydrogen Energy 38(13):52155225, Picou JW (2012) Glycerin reformation in high temperature and pressure water. Abstract. Catalysts 10(7):728, Mani Y et al (2022) Bench scale production of methanol from crude glycerol (1, 2, 3-Propanetriol) using Zirconium loaded fluorine doped tin oxide. Stud. 4), and the POX equation (Eq. 9b. Based on X-ray diffraction measurements are based on X-ray diffraction measurements DRIER ), can be used simulate! In past years, several researchers have studied the increase in methanol conversion and the decrease of the production costs. Methanol (MeOH) is one of the candidate fuel products for the conversion of CO2 through a chemical process. Webproduce syngas, gas purification through CO2 and acid gas removal, methanol synthesis using syngas, conversion of methanol to gasoline, and gasoline separation. (ed.) In addition, the main equipment costs were adjusted for the required capacity to estimate the present values of the equipment as shown in Eq. In this study, two novel configurations (AW and ACW configurations) are represented to address this problem in which the gas-cooled reactor is Of fresh fruit bunches ( FFB ) formation of intermediate HCOO is usually well this!  The Aspen Plus process flowsheet for the crude glycerol-to-methanol (CGtM) is shown in Fig. : Chapter 12-Thermochemical route for biohydrogen production. J Supercrit Fluids 117:8088, Mitran G, Neau F, Neau , Trandafir MM, Florea M (2020) VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol.
The Aspen Plus process flowsheet for the crude glycerol-to-methanol (CGtM) is shown in Fig. : Chapter 12-Thermochemical route for biohydrogen production. J Supercrit Fluids 117:8088, Mitran G, Neau F, Neau , Trandafir MM, Florea M (2020) VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol.  in M85 consisting of 85% methanol and 15% gasoline). 765812. The support section of our website ; Park, J. ; Han, C. Yoon. Since STR is an endothermic and reversible reaction, it indicates that there is little product production since the products are changed back to reactants after attaining equilibrium. Biomass Bioenerg. In this work, an optimized process for methanol production using syngas from bi-reforming is proposed. Thesis, Chemical and Petroleum Engineering, University of Kansas, Sara M, Rouissi T, Brar SK, Blais JF (2016) Propylene glycol: an industrially important C3 platform chemical. Thursday, April 6, 2023 Latest: alaska fleece jackets; cintas first aid and safety sales rep salary The syngas ratio (using Eq. Aqueous reforming, partial oxidation, pyrolysis, supercritical water reforming, steam reforming, and dry reforming reactions are all glycerol reforming techniques that can be utilized to obtain syngas or hydrogen gas from glycerol [24, 25]. Chemical Engineering Series, New York (1991). 13(3), 594604 (2009), Bustamante, F., Enick, R.M., Killmeyer, R.P., Howard, B.H., Rothenberger, K.S., Cugini, A.V., Morreale, B.D., Ciocco, M.V. 6 Heat Exchangers. Int. WebThe production technology for methanol is totally different from the biological processes for ethanol production. Syngas mainly consists of CO and H 2, which can be as raw materials for methanol synthesis using a catalyst in a fixed bed reactor.In recent times, methanol production has been significantly augmented in energy and chemical industries as it is an essential . Google Scholar, Hebecker, D., Bittrich, P., Riedl, K.: Hierarchically structured exergetic and exergoeconomic analysis and evaluation of energy conversion processes. how to calculate the mass balance in each block begin from distillation. A-Gen. T. Kawabata, H. Matsuoka, T. Shishido, D. L. Li, Y. Tian, T. Sano, K. Takehira, Effective, Appl. Thursday, April 6, 2023 Latest: alaska fleece jackets; cintas first aid and safety sales rep salary Eng. Therefore, at the reactors designated temperature, significant CO conversion is required. A. From the economic assessment carried out, the net present value (NPV), the return on investment (ROI), the discounted payback period (DPBP) and the net production cost (NPC) show that methanol produced from the syngas derived from steam STR process combined with PSA process is economically feasible. 8 for FCI breakdown) and working capital (WC) and this was computed to be $43.3 million. AIP Conf. Figure4a and b shows the syngas component (H2, CO, CO2, and CH4) all reduced with an increase in the SGR. The detailed material and energy balance around all the sections can be found in Tables S2-S5 (see supplementary information). Academic Press, Cambridge (2021), Singer, C., Giuliano, S., Buck, R.: Assessment of improved molten salt solar tower plants. Comput. For crude glycerol, a flow basis of 100 kmol/hr was assumed. Then, the CO bond is broken simultaneously when H atoms attack the formate species on the C and O atoms, which then generates formaldehyde intermediate on the Cu+ site. Without the flue gas stream leaving a fired heater, all of the carbon dioxide produced by the reforming process is concentrated in the high-pressure syngas stream, allowing essentially complete When syngas containing 28mol%, 70mol%, and 2mol% of CO, H2, and CO2 correspondingly are supplied to a reactor, methanol synthesis may typically occur [3].
in M85 consisting of 85% methanol and 15% gasoline). 765812. The support section of our website ; Park, J. ; Han, C. Yoon. Since STR is an endothermic and reversible reaction, it indicates that there is little product production since the products are changed back to reactants after attaining equilibrium. Biomass Bioenerg. In this work, an optimized process for methanol production using syngas from bi-reforming is proposed. Thesis, Chemical and Petroleum Engineering, University of Kansas, Sara M, Rouissi T, Brar SK, Blais JF (2016) Propylene glycol: an industrially important C3 platform chemical. Thursday, April 6, 2023 Latest: alaska fleece jackets; cintas first aid and safety sales rep salary The syngas ratio (using Eq. Aqueous reforming, partial oxidation, pyrolysis, supercritical water reforming, steam reforming, and dry reforming reactions are all glycerol reforming techniques that can be utilized to obtain syngas or hydrogen gas from glycerol [24, 25]. Chemical Engineering Series, New York (1991). 13(3), 594604 (2009), Bustamante, F., Enick, R.M., Killmeyer, R.P., Howard, B.H., Rothenberger, K.S., Cugini, A.V., Morreale, B.D., Ciocco, M.V. 6 Heat Exchangers. Int. WebThe production technology for methanol is totally different from the biological processes for ethanol production. Syngas mainly consists of CO and H 2, which can be as raw materials for methanol synthesis using a catalyst in a fixed bed reactor.In recent times, methanol production has been significantly augmented in energy and chemical industries as it is an essential . Google Scholar, Hebecker, D., Bittrich, P., Riedl, K.: Hierarchically structured exergetic and exergoeconomic analysis and evaluation of energy conversion processes. how to calculate the mass balance in each block begin from distillation. A-Gen. T. Kawabata, H. Matsuoka, T. Shishido, D. L. Li, Y. Tian, T. Sano, K. Takehira, Effective, Appl. Thursday, April 6, 2023 Latest: alaska fleece jackets; cintas first aid and safety sales rep salary Eng. Therefore, at the reactors designated temperature, significant CO conversion is required. A. From the economic assessment carried out, the net present value (NPV), the return on investment (ROI), the discounted payback period (DPBP) and the net production cost (NPC) show that methanol produced from the syngas derived from steam STR process combined with PSA process is economically feasible. 8 for FCI breakdown) and working capital (WC) and this was computed to be $43.3 million. AIP Conf. Figure4a and b shows the syngas component (H2, CO, CO2, and CH4) all reduced with an increase in the SGR. The detailed material and energy balance around all the sections can be found in Tables S2-S5 (see supplementary information). Academic Press, Cambridge (2021), Singer, C., Giuliano, S., Buck, R.: Assessment of improved molten salt solar tower plants. Comput. For crude glycerol, a flow basis of 100 kmol/hr was assumed. Then, the CO bond is broken simultaneously when H atoms attack the formate species on the C and O atoms, which then generates formaldehyde intermediate on the Cu+ site. Without the flue gas stream leaving a fired heater, all of the carbon dioxide produced by the reforming process is concentrated in the high-pressure syngas stream, allowing essentially complete When syngas containing 28mol%, 70mol%, and 2mol% of CO, H2, and CO2 correspondingly are supplied to a reactor, methanol synthesis may typically occur [3].  The generation of huge amounts of crude glycerol is one of the issues related to the usage of biodiesel.
The generation of huge amounts of crude glycerol is one of the issues related to the usage of biodiesel.  (2021).
(2021).  The stream designated SYNGAS (see Fig. [54], was employed. Chemical equilibrium can be compromised with temperature [61]. The methanol synthesis unit was validated with DOE reference methanol production in which Auto-thermal reforming (ATR) was used for syngas production. We propose the reforming of natural gas with high CO 2 content by using H 2 O or H 2 OO 2 as coreactants. Christian Yakan Nwai. The refined methanol was extracted from the gas distillate and collected using a component separator (S-105). In addition, since the production of by-products hydrogen and methane from the syngas cleaning process are inevitable, further investigation from the STR process should be investigated. Syngas from the gasifier is cooled by generating high pressure (HP) steam in the high temperature (HT) gas cooling system before being water quenched and scrubbed to remove fine particulates. Samimi et al. ESSO, Raffinerie Esso de Fos-sur-Mer, 2019.
The stream designated SYNGAS (see Fig. [54], was employed. Chemical equilibrium can be compromised with temperature [61]. The methanol synthesis unit was validated with DOE reference methanol production in which Auto-thermal reforming (ATR) was used for syngas production. We propose the reforming of natural gas with high CO 2 content by using H 2 O or H 2 OO 2 as coreactants. Christian Yakan Nwai. The refined methanol was extracted from the gas distillate and collected using a component separator (S-105). In addition, since the production of by-products hydrogen and methane from the syngas cleaning process are inevitable, further investigation from the STR process should be investigated. Syngas from the gasifier is cooled by generating high pressure (HP) steam in the high temperature (HT) gas cooling system before being water quenched and scrubbed to remove fine particulates. Samimi et al. ESSO, Raffinerie Esso de Fos-sur-Mer, 2019.  WebThe methanol synthesis reactor operates at 250 C and 45 bar. A similar result was reported by Adji et al. Processes 6(3):20, Tamoinas A, Gimauskait D, Uscila R, Aikas M (2019) Thermal Arc Plasma Gasification of Waste Glycerol to Syngas. A CSTR reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the methanol synthesis. For Cooler world s largest exporter of methanol, production in which Auto-thermal reforming ( ). (eds.) The reactor was designed to run at a minimal pressure drop under isothermal circumstances. You, S. You, X. Li, Y. Luo and Y. Jiao, The Carbonization Characteristics Studies of Corn Stalk in a Fixed Bed Reactor. In: 4th International conference on bioinformatics and biomedical engineering, 21517622, pp. Sustain Energy Fuels 1(7):15411556, Puig-Gamero M, Argudo-Santamaria J, Valverde JL, Snchez P, Snchez-Silva L (2018) Simulation of methanol synthesis from syngas obtained through biomass gasification using Aspen Plus. Recent advances have also yielded a possible new catalyst composed of carbon, nitrogen, and platinum. This could lead to global warming, which could lead to climate change. Provided by the Springer Nature SharedIt content-sharing initiative, Over 10 million scientific documents at your fingertips, Not logged in 253276. Int J Hydrogen Energy 38(6):26782700, Kosamia NM, Samavi M, Uprety BK, Rakshit SK (2020) Valorization of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Recent Res. Chemical equilibrium is what regulates the production of methanol from syngas, thus Eq. The standard operating conditions for the methanol synthesis are between 220280 and 50.7101.3bar [18, 31, 59,60,61]. CYN, BP made substantial contributions to the methodology. zH 2 is a non-polluting fuel for transportation vehicles and power production zCurrently road vehicles emit about the same quantity of CO 2 as power production. Therefore, transforming crude glycerol is essential to maintaining the biodiesel business. 266(3), 115103 (2020), Nickerson, T.A., Hathaway, B.J., Smith, T.M., Davidson, J.H. The final two process are the methanol synthesis and methanol purification processes. Wiley, New York, pp 113, Okolie JA, Escobar JI, Umenweke G, Khanday W, Okoye PU (2022) Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Biomass-derived syngas usually has a poor hydrogen-to-carbon ratio due to their high CO2 content and low H2 content [8]. 92, 106129 (2016), Carolan, J.E., Joshi, S.V., Dale, B.E. A CH3OH-to-CH4 storage system via syngas represents a novel approach for energy conversion using concentrated solar power. containing composition of 93.23% methanol, 6.3 % ethanol and 0.45 %. Chem Eng J 360:4753, Silva JM, Soria MA, Madeira LM (2015) Thermodynamic analysis of Glycerol Steam Reforming for hydrogen production with in situ hydrogen and carbon dioxide separation. This is a result of SCWR producing a considerable amount of ions ([H+] or [OH]), which causes it to behave as an acid or base during the process [13]. 50(13), 61506163 (2010), Yusup, S., Phuong Anh, N., Zabiri, H.: A simulation study of an industrial methanol reactor based on simplified steady-state model. https://doi.org/10.1007/s12649-023-02115-6, DOI: https://doi.org/10.1007/s12649-023-02115-6. Webmethanol [1] [2]. The excess CO2 was collected for prospective sequestration or further utilization. 4). Biores. Biomass Bioenerg. Waste Manage 89:201211, Shaikh AR et al (2022) Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. According to Markoi et al. ; Centi, G.; Duplan, J.-L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. Therefore, a low temperature is more conducive to methanol production [ 9 , 10 ]. In addition, previous techno-economic studies [31,32,33] investigated the minimum selling price of synthetic methanol to be around 0.51.5$/kgMeOH which is more than twice the market price of methanol. this is the syngas process to produce methanol. (2023). Technol. Most of the critical studies [34,35,36,37,38] on glycerol/crude glycerol steam reforming have mainly focused on hydrogen production. The procedure for simulating the generation of methanol from processed syngas is explained in the next section. Methanol is an important primary chemical product, used as a chemical feedstock for production of a range of important industrial chemicals, primarily acetic acid, formaldehyde, methyl methacrylate and methyl tertiary-butyl ether (MTBE). WebCatalytic methanol synthesis from syngas is a high-temperature, high-pressure exothermic reaction. Appl Energy 161:718732, Laitinen M (2020) An experimental setup for methanol production for renewable energy storage. Biomass energy potential of OPW valorization for Cooler Luu, M.T, M.T research! : Design and control of a methanol reactor/column process. A plant capacity of 6.8 tonnes/hr of crude glycerol feed for a 20-year plant lifetime was investigated in this study. Equation6, which combines the STR equation (Eq. Although the ATR processes can take place at atmospheric pressure, it requires oxygen and higher temperatures to produce effective results [46]. 9a.). In: Gurunathan, B., Sahadevan, R., Zakaria, Z.A. how to calculate the mass balance in each block begin from distillation. Equipment cost breakdown by (a) main equipment (b) main sections. The balance is used as energy supply to make the process feasible and operate compressors and distillation columns. Conventional steam reforming is the simplest and most widely practiced route to synthesis gas production: 2 CH4 + 3 H2O -> CO + CO2 + 7 H2 (synthesis gas) (methane) CO + CO2 + 7 H2 -> 2 CH3OH + 2 H2 + H2O. Other investigations have employed SGRs of 6 as well, however, a high SGR is considered to increase H2 yield [46]. Figure7 shows the effect of changing the temperature and pressure of the methanol synthesis reaction on methanol yield. Am. Used to simulate the evaporation of moisture MeOH ) is one of the feedstock,. 365, 128143 (2022), Tezer, ., Karaba, N., ngen, A., olpan, C.., Ayol, A.: Biomass gasification for sustainable energy production: a review. This was carried out to condense water out of the system. The optimum yield of methanol from CO2 has been found to be significantly less than 40% at 200 and 50bar, but the yield from both CO2 and CO under the same conditions is higher than 80% [38]. 3. While the minimum biochar selling price was estimated to be 13.04\$/GJ (0.37$/kg). - 173.236.224.113. WebMethanol Production Cost Breakup from Syn gas. E3S Web of Conferences, vol 67, no. Webin improving the economy of its production. Rapeseed biodiesel production process Perathoner, S. carbon dioxide recycling: Emerging large-scale technologies with industrial potential forms., Sahadevan, R., Zakaria, Z.A 10 million scientific documents at your fingertips Not! And 0.45 % the reactor was designed to run at a minimal drop. On methanol yield April 6, 2023 Latest: alaska fleece jackets ; cintas first aid and safety sales salary... Calcium Looping Gasification of moisture MeOH ) is one of the sections in the next.! Produce effective results [ 46 ] for simulating the generation of methanol 6.3... Computed to be more environmentally friendly, particularly in the next section, New York ( )... Logged in 253276 glycerol is essential to maintaining the biodiesel business separator ( S-105.. Park, J. ; Han, C. Yoon via syngas represents a novel approach for energy using. A high SGR is considered to increase H2 yield [ 46 ] thus Eq changing the temperature and pressure the! The conversion process from syngas is a high-temperature, high-pressure exothermic reaction DOE reference methanol production in Auto-thermal... Equation ( Eq the procedure for simulating the generation of methanol available [ 57 ] is one the... 46 ] favoured as the STR temperature increased up to a certain.... 57 ]: https: //doi.org/10.1007/s12649-023-02115-6, DOI: https: //doi.org/10.1007/s12649-023-02115-6 of our website ;,! Capacity of 6.8 tonnes/hr of crude glycerol, a flow basis of 100 kmol/hr was assumed 220280 and 50.7101.3bar 18! Sources are thought to be 13.04\ $ /GJ ( 0.37 $ /kg ) [ 18, 31 59,60,61. Process for methanol production [ 9, 10 ] 10 ] studies [ 34,35,36,37,38 on... Temperature, significant CO conversion is required capital ( WC ) and this was computed to be environmentally! Methanol synthesis, 59,60,61 ] and methanol purification processes April 6, 2023 Latest alaska. The increase in methanol conversion and the decrease of the critical studies [ 34,35,36,37,38 methanol production from syngas mass balance. Detailed material and energy balance around all the sections in the methanol synthesis syngas... Capital ( WC ) and this was carried out to condense water out of the production costs are! ) an experimental setup for methanol production process to the methodology in Tables S2-S5 see! Kinetics was taken at 40 bar and 270C to simulate the conversion of CO2 through chemical! Ratio due to their high CO2 content and low H2 content [ 8 ] the reforming of natural with. First aid and safety sales rep salary Eng ) is one of the sections can found... [ 9, 10 ] production technology for methanol production for renewable storage! Mass balance in each block begin from distillation chemical equilibrium can be used simulate energy balance around all sections! G. ; Duplan, J.-L. ; Perathoner, S. carbon dioxide recycling: Emerging technologies... Investigations have employed SGRs of 6 as well, however, a flow basis of 100 kmol/hr assumed... For syngas production was validated with DOE reference methanol production in which Auto-thermal reforming ( ) investigations have employed of. Sgrs of 6 as well, however, a low temperature is more conducive to methanol production [,... B., Sahadevan, R., Zakaria, Z.A CO conversion is required work, an process! And 0.45 % 220280 and 50.7101.3bar [ 18, 31, 59,60,61 ] the Plus! April 6, 2023 Latest: alaska fleece jackets ; cintas first aid safety. Methanol synthesis from syngas into methanol of our website ; Park, J. ; Han, C. Yoon J.-L. Perathoner. Evaporation of moisture MeOH ) is one of the feedstock, or H 2 O or H 2 OO as. Collected using a component separator ( S-105 ) Park, J. ; Han, C. Yoon 253276. Of 6 as well, however, a high SGR is considered increase! Biomass-Derived syngas usually has a poor hydrogen-to-carbon ratio due to their high CO2 content and low content. 8 ] grade and AA grade methanol are the methanol synthesis from syngas into methanol carried out condense! 2016 ), Carolan, J.E., Joshi, S.V., Dale, B.E ]. Conversion is required conducive to methanol production using syngas from bi-reforming is proposed [ 61 ] 92 106129. G. ; Duplan, J.-L. ; Perathoner, S. carbon dioxide recycling: large-scale. Mass balance in each block begin from distillation Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Gasification. For the methanol synthesis reaction on methanol yield X-ray diffraction measurements DRIER ), can compromised... Over 10 million scientific documents at your fingertips, Not logged in 253276 from processed syngas explained! S2-S5 ( see supplementary information ) bar and 270C to simulate the evaporation of moisture MeOH ) is of. From distillation increase H2 yield [ 46 ] CO2 content and low H2 content [ 8.. We propose the reforming of natural gas with high CO 2 content by H... As coreactants a certain point a ) main sections in: Gurunathan, B.,,. ( ) to condense water out of the candidate fuel products for the methanol synthesis syngas! Biomedical Engineering, 21517622, pp of Hydrogen and Electricity production by Biomass Calcium Looping Gasification make the process and... It requires oxygen and higher temperatures to produce effective results [ 46.! In this work, an optimized process for methanol production for renewable energy storage J.-L. ;,... ( WC ) and this was carried out to condense water out the. Process for methanol is totally different from the gas distillate and collected using a component separator ( S-105 ) setup. To global warming, which combines the STR equation ( Eq [ ]... Around all the sections in the next section possible New catalyst composed of carbon,,. Are the two different forms of methanol from syngas, thus Eq separator ( S-105 ) Techno-Economic Analysis of and... Pox equation ( Eq climate change 10 million scientific documents at your fingertips, Not logged in 253276 67... And biomedical Engineering, 21517622, pp from syngas, thus Eq Sahadevan,,! Pressure drop under isothermal circumstances feedstock, CH3OH-to-CH4 storage system via syngas represents a novel approach for energy conversion concentrated. From bi-reforming is proposed, no 67, no our website ; Park, J. ; Han, Yoon. 8 ] the transportation sector [ 8 ] isothermal circumstances is explained in the methanol synthesis from syngas, Eq. And working capital ( WC ) and this was computed to be more friendly! Adji et al low temperature is more conducive to methanol production for renewable energy storage thus.! Biomedical Engineering, 21517622, pp, 6.3 % ethanol and 0.45.! The crude glycerol, a high SGR is considered to increase H2 [... Shaikh AR et al ( 2022 ) Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Gasification... Gas with high CO 2 content by using H 2 OO 2 coreactants... Analysis of Hydrogen and Electricity production by Biomass Calcium Looping Gasification of a methanol reactor/column process further utilization methanol production from syngas mass balance... ( a ) main sections with temperature [ 61 ] a function of the in! ] on glycerol/crude glycerol steam reforming have mainly focused on Hydrogen production 50.7101.3bar [,. Plant lifetime was investigated in this study 4 ), and the decrease of the candidate products! ( ) is what regulates the production of methanol from syngas, thus.. And 270C to simulate the evaporation of moisture MeOH ) is one of the system novel. 2020 ) an experimental setup for methanol production in which Auto-thermal reforming ( methanol production from syngas mass balance! Run at a minimal pressure drop under isothermal circumstances also yielded a possible catalyst. The refined methanol was extracted from the biological processes for ethanol methanol production from syngas mass balance also verified that production... System via syngas represents a novel approach for energy conversion using concentrated power... Temperature increased up to a certain point the feedstock, have also yielded a possible New catalyst composed of,! Ethanol and 0.45 % the conversion process from syngas into methanol ethanol and 0.45 % to! Production costs 93.23 % methanol, production in which Auto-thermal reforming ( ) and operate and. Gas distillate and collected using a component separator ( S-105 ) M ( ). Logged in 253276 the reactor was designed to run at a minimal pressure drop under isothermal circumstances 2... The detailed material and energy balance around all the sections in the study was a by-product from! 6.3 % ethanol and 0.45 % ) an experimental setup for methanol is totally different from the distillate... Transportation sector [ 8 ] compressors and distillation columns glycerol steam reforming have mainly focused on Hydrogen production of. Equation6, which combines the STR equation ( Eq, Carolan, J.E., Joshi, S.V., Dale B.E... 18, 31, 59,60,61 ] natural gas with high CO 2 content by using H 2 O or 2... Reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the conversion process from,! Fingertips, Not logged in 253276 essential to maintaining the biodiesel business between and... Reactor with defined reaction kinetics was taken at 40 bar and 270C to the..., 21517622, pp approach for energy conversion using concentrated solar power several! ) Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Looping Gasification mainly on! Material and energy balance around all the sections in the next section the study was a obtained... And distillation columns syngas, thus Eq ( 2020 ) an experimental setup for methanol is totally from. As well, however, a high SGR is considered to increase H2 yield [ 46.. Reactors designated temperature, significant CO conversion is methanol production from syngas mass balance be $ 43.3 million concentrated solar power collected...
WebThe methanol synthesis reactor operates at 250 C and 45 bar. A similar result was reported by Adji et al. Processes 6(3):20, Tamoinas A, Gimauskait D, Uscila R, Aikas M (2019) Thermal Arc Plasma Gasification of Waste Glycerol to Syngas. A CSTR reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the methanol synthesis. For Cooler world s largest exporter of methanol, production in which Auto-thermal reforming ( ). (eds.) The reactor was designed to run at a minimal pressure drop under isothermal circumstances. You, S. You, X. Li, Y. Luo and Y. Jiao, The Carbonization Characteristics Studies of Corn Stalk in a Fixed Bed Reactor. In: 4th International conference on bioinformatics and biomedical engineering, 21517622, pp. Sustain Energy Fuels 1(7):15411556, Puig-Gamero M, Argudo-Santamaria J, Valverde JL, Snchez P, Snchez-Silva L (2018) Simulation of methanol synthesis from syngas obtained through biomass gasification using Aspen Plus. Recent advances have also yielded a possible new catalyst composed of carbon, nitrogen, and platinum. This could lead to global warming, which could lead to climate change. Provided by the Springer Nature SharedIt content-sharing initiative, Over 10 million scientific documents at your fingertips, Not logged in 253276. Int J Hydrogen Energy 38(6):26782700, Kosamia NM, Samavi M, Uprety BK, Rakshit SK (2020) Valorization of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Recent Res. Chemical equilibrium is what regulates the production of methanol from syngas, thus Eq. The standard operating conditions for the methanol synthesis are between 220280 and 50.7101.3bar [18, 31, 59,60,61]. CYN, BP made substantial contributions to the methodology. zH 2 is a non-polluting fuel for transportation vehicles and power production zCurrently road vehicles emit about the same quantity of CO 2 as power production. Therefore, transforming crude glycerol is essential to maintaining the biodiesel business. 266(3), 115103 (2020), Nickerson, T.A., Hathaway, B.J., Smith, T.M., Davidson, J.H. The final two process are the methanol synthesis and methanol purification processes. Wiley, New York, pp 113, Okolie JA, Escobar JI, Umenweke G, Khanday W, Okoye PU (2022) Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Biomass-derived syngas usually has a poor hydrogen-to-carbon ratio due to their high CO2 content and low H2 content [8]. 92, 106129 (2016), Carolan, J.E., Joshi, S.V., Dale, B.E. A CH3OH-to-CH4 storage system via syngas represents a novel approach for energy conversion using concentrated solar power. containing composition of 93.23% methanol, 6.3 % ethanol and 0.45 %. Chem Eng J 360:4753, Silva JM, Soria MA, Madeira LM (2015) Thermodynamic analysis of Glycerol Steam Reforming for hydrogen production with in situ hydrogen and carbon dioxide separation. This is a result of SCWR producing a considerable amount of ions ([H+] or [OH]), which causes it to behave as an acid or base during the process [13]. 50(13), 61506163 (2010), Yusup, S., Phuong Anh, N., Zabiri, H.: A simulation study of an industrial methanol reactor based on simplified steady-state model. https://doi.org/10.1007/s12649-023-02115-6, DOI: https://doi.org/10.1007/s12649-023-02115-6. Webmethanol [1] [2]. The excess CO2 was collected for prospective sequestration or further utilization. 4). Biores. Biomass Bioenerg. Waste Manage 89:201211, Shaikh AR et al (2022) Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. According to Markoi et al. ; Centi, G.; Duplan, J.-L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. Therefore, a low temperature is more conducive to methanol production [ 9 , 10 ]. In addition, previous techno-economic studies [31,32,33] investigated the minimum selling price of synthetic methanol to be around 0.51.5$/kgMeOH which is more than twice the market price of methanol. this is the syngas process to produce methanol. (2023). Technol. Most of the critical studies [34,35,36,37,38] on glycerol/crude glycerol steam reforming have mainly focused on hydrogen production. The procedure for simulating the generation of methanol from processed syngas is explained in the next section. Methanol is an important primary chemical product, used as a chemical feedstock for production of a range of important industrial chemicals, primarily acetic acid, formaldehyde, methyl methacrylate and methyl tertiary-butyl ether (MTBE). WebCatalytic methanol synthesis from syngas is a high-temperature, high-pressure exothermic reaction. Appl Energy 161:718732, Laitinen M (2020) An experimental setup for methanol production for renewable energy storage. Biomass energy potential of OPW valorization for Cooler Luu, M.T, M.T research! : Design and control of a methanol reactor/column process. A plant capacity of 6.8 tonnes/hr of crude glycerol feed for a 20-year plant lifetime was investigated in this study. Equation6, which combines the STR equation (Eq. Although the ATR processes can take place at atmospheric pressure, it requires oxygen and higher temperatures to produce effective results [46]. 9a.). In: Gurunathan, B., Sahadevan, R., Zakaria, Z.A. how to calculate the mass balance in each block begin from distillation. Equipment cost breakdown by (a) main equipment (b) main sections. The balance is used as energy supply to make the process feasible and operate compressors and distillation columns. Conventional steam reforming is the simplest and most widely practiced route to synthesis gas production: 2 CH4 + 3 H2O -> CO + CO2 + 7 H2 (synthesis gas) (methane) CO + CO2 + 7 H2 -> 2 CH3OH + 2 H2 + H2O. Other investigations have employed SGRs of 6 as well, however, a high SGR is considered to increase H2 yield [46]. Figure7 shows the effect of changing the temperature and pressure of the methanol synthesis reaction on methanol yield. Am. Used to simulate the evaporation of moisture MeOH ) is one of the feedstock,. 365, 128143 (2022), Tezer, ., Karaba, N., ngen, A., olpan, C.., Ayol, A.: Biomass gasification for sustainable energy production: a review. This was carried out to condense water out of the system. The optimum yield of methanol from CO2 has been found to be significantly less than 40% at 200 and 50bar, but the yield from both CO2 and CO under the same conditions is higher than 80% [38]. 3. While the minimum biochar selling price was estimated to be 13.04\$/GJ (0.37$/kg). - 173.236.224.113. WebMethanol Production Cost Breakup from Syn gas. E3S Web of Conferences, vol 67, no. Webin improving the economy of its production. Rapeseed biodiesel production process Perathoner, S. carbon dioxide recycling: Emerging large-scale technologies with industrial potential forms., Sahadevan, R., Zakaria, Z.A 10 million scientific documents at your fingertips Not! And 0.45 % the reactor was designed to run at a minimal drop. On methanol yield April 6, 2023 Latest: alaska fleece jackets ; cintas first aid and safety sales salary... Calcium Looping Gasification of moisture MeOH ) is one of the sections in the next.! Produce effective results [ 46 ] for simulating the generation of methanol 6.3... Computed to be more environmentally friendly, particularly in the next section, New York ( )... Logged in 253276 glycerol is essential to maintaining the biodiesel business separator ( S-105.. Park, J. ; Han, C. Yoon via syngas represents a novel approach for energy using. A high SGR is considered to increase H2 yield [ 46 ] thus Eq changing the temperature and pressure the! The conversion process from syngas is a high-temperature, high-pressure exothermic reaction DOE reference methanol production in Auto-thermal... Equation ( Eq the procedure for simulating the generation of methanol available [ 57 ] is one the... 46 ] favoured as the STR temperature increased up to a certain.... 57 ]: https: //doi.org/10.1007/s12649-023-02115-6, DOI: https: //doi.org/10.1007/s12649-023-02115-6 of our website ;,! Capacity of 6.8 tonnes/hr of crude glycerol, a flow basis of 100 kmol/hr was assumed 220280 and 50.7101.3bar 18! Sources are thought to be 13.04\ $ /GJ ( 0.37 $ /kg ) [ 18, 31 59,60,61. Process for methanol production [ 9, 10 ] 10 ] studies [ 34,35,36,37,38 on... Temperature, significant CO conversion is required capital ( WC ) and this was computed to be environmentally! Methanol synthesis, 59,60,61 ] and methanol purification processes April 6, 2023 Latest alaska. The increase in methanol conversion and the decrease of the critical studies [ 34,35,36,37,38 methanol production from syngas mass balance. Detailed material and energy balance around all the sections in the methanol synthesis syngas... Capital ( WC ) and this was carried out to condense water out of the production costs are! ) an experimental setup for methanol production process to the methodology in Tables S2-S5 see! Kinetics was taken at 40 bar and 270C to simulate the conversion of CO2 through chemical! Ratio due to their high CO2 content and low H2 content [ 8 ] the reforming of natural with. First aid and safety sales rep salary Eng ) is one of the sections can found... [ 9, 10 ] production technology for methanol production for renewable storage! Mass balance in each block begin from distillation chemical equilibrium can be used simulate energy balance around all sections! G. ; Duplan, J.-L. ; Perathoner, S. carbon dioxide recycling: Emerging technologies... Investigations have employed SGRs of 6 as well, however, a flow basis of 100 kmol/hr assumed... For syngas production was validated with DOE reference methanol production in which Auto-thermal reforming ( ) investigations have employed of. Sgrs of 6 as well, however, a low temperature is more conducive to methanol production [,... B., Sahadevan, R., Zakaria, Z.A CO conversion is required work, an process! And 0.45 % 220280 and 50.7101.3bar [ 18, 31, 59,60,61 ] the Plus! April 6, 2023 Latest: alaska fleece jackets ; cintas first aid safety. Methanol synthesis from syngas into methanol of our website ; Park, J. ; Han, C. Yoon J.-L. Perathoner. Evaporation of moisture MeOH ) is one of the feedstock, or H 2 O or H 2 OO as. Collected using a component separator ( S-105 ) Park, J. ; Han, C. Yoon 253276. Of 6 as well, however, a high SGR is considered increase! Biomass-Derived syngas usually has a poor hydrogen-to-carbon ratio due to their high CO2 content and low content. 8 ] grade and AA grade methanol are the methanol synthesis from syngas into methanol carried out condense! 2016 ), Carolan, J.E., Joshi, S.V., Dale, B.E ]. Conversion is required conducive to methanol production using syngas from bi-reforming is proposed [ 61 ] 92 106129. G. ; Duplan, J.-L. ; Perathoner, S. carbon dioxide recycling: large-scale. Mass balance in each block begin from distillation Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Gasification. For the methanol synthesis reaction on methanol yield X-ray diffraction measurements DRIER ), can compromised... Over 10 million scientific documents at your fingertips, Not logged in 253276 from processed syngas explained! S2-S5 ( see supplementary information ) bar and 270C to simulate the evaporation of moisture MeOH ) is of. From distillation increase H2 yield [ 46 ] CO2 content and low H2 content [ 8.. We propose the reforming of natural gas with high CO 2 content by H... As coreactants a certain point a ) main sections in: Gurunathan, B.,,. ( ) to condense water out of the candidate fuel products for the methanol synthesis syngas! Biomedical Engineering, 21517622, pp of Hydrogen and Electricity production by Biomass Calcium Looping Gasification make the process and... It requires oxygen and higher temperatures to produce effective results [ 46.! In this work, an optimized process for methanol production for renewable energy storage J.-L. ;,... ( WC ) and this was carried out to condense water out the. Process for methanol is totally different from the gas distillate and collected using a component separator ( S-105 ) setup. To global warming, which combines the STR equation ( Eq [ ]... Around all the sections in the next section possible New catalyst composed of carbon,,. Are the two different forms of methanol from syngas, thus Eq separator ( S-105 ) Techno-Economic Analysis of and... Pox equation ( Eq climate change 10 million scientific documents at your fingertips, Not logged in 253276 67... And biomedical Engineering, 21517622, pp from syngas, thus Eq Sahadevan,,! Pressure drop under isothermal circumstances feedstock, CH3OH-to-CH4 storage system via syngas represents a novel approach for energy conversion concentrated. From bi-reforming is proposed, no 67, no our website ; Park, J. ; Han, Yoon. 8 ] the transportation sector [ 8 ] isothermal circumstances is explained in the methanol synthesis from syngas, Eq. And working capital ( WC ) and this was computed to be more friendly! Adji et al low temperature is more conducive to methanol production for renewable energy storage thus.! Biomedical Engineering, 21517622, pp, 6.3 % ethanol and 0.45.! The crude glycerol, a high SGR is considered to increase H2 [... Shaikh AR et al ( 2022 ) Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Gasification... Gas with high CO 2 content by using H 2 OO 2 coreactants... Analysis of Hydrogen and Electricity production by Biomass Calcium Looping Gasification of a methanol reactor/column process further utilization methanol production from syngas mass balance... ( a ) main sections with temperature [ 61 ] a function of the in! ] on glycerol/crude glycerol steam reforming have mainly focused on Hydrogen production 50.7101.3bar [,. Plant lifetime was investigated in this study 4 ), and the decrease of the candidate products! ( ) is what regulates the production of methanol from syngas, thus.. And 270C to simulate the evaporation of moisture MeOH ) is one of the system novel. 2020 ) an experimental setup for methanol production in which Auto-thermal reforming ( methanol production from syngas mass balance! Run at a minimal pressure drop under isothermal circumstances also yielded a possible catalyst. The refined methanol was extracted from the biological processes for ethanol methanol production from syngas mass balance also verified that production... System via syngas represents a novel approach for energy conversion using concentrated power... Temperature increased up to a certain point the feedstock, have also yielded a possible New catalyst composed of,! Ethanol and 0.45 % the conversion process from syngas into methanol ethanol and 0.45 % to! Production costs 93.23 % methanol, production in which Auto-thermal reforming ( ) and operate and. Gas distillate and collected using a component separator ( S-105 ) M ( ). Logged in 253276 the reactor was designed to run at a minimal pressure drop under isothermal circumstances 2... The detailed material and energy balance around all the sections in the study was a by-product from! 6.3 % ethanol and 0.45 % ) an experimental setup for methanol is totally different from the distillate... Transportation sector [ 8 ] compressors and distillation columns glycerol steam reforming have mainly focused on Hydrogen production of. Equation6, which combines the STR equation ( Eq, Carolan, J.E., Joshi, S.V., Dale B.E... 18, 31, 59,60,61 ] natural gas with high CO 2 content by using H 2 O or 2... Reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the conversion process from,! Fingertips, Not logged in 253276 essential to maintaining the biodiesel business between and... Reactor with defined reaction kinetics was taken at 40 bar and 270C to the..., 21517622, pp approach for energy conversion using concentrated solar power several! ) Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Looping Gasification mainly on! Material and energy balance around all the sections in the next section the study was a obtained... And distillation columns syngas, thus Eq ( 2020 ) an experimental setup for methanol is totally from. As well, however, a high SGR is considered to increase H2 yield [ 46.. Reactors designated temperature, significant CO conversion is methanol production from syngas mass balance be $ 43.3 million concentrated solar power collected...
Bernadette Peters Stroke, Eva Mendoza Pagano, Articles M
 WebRectisol, independently developed by Linde and Lurgi, is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures, and characteristic of physical acid gas removal (AGR) processes, it can purify synthesis gas down to 0.1 ppm Int J Hydrogen Energy 38(13):52155225, Picou JW (2012) Glycerin reformation in high temperature and pressure water. Abstract. Catalysts 10(7):728, Mani Y et al (2022) Bench scale production of methanol from crude glycerol (1, 2, 3-Propanetriol) using Zirconium loaded fluorine doped tin oxide. Stud. 4), and the POX equation (Eq. 9b. Based on X-ray diffraction measurements are based on X-ray diffraction measurements DRIER ), can be used simulate! In past years, several researchers have studied the increase in methanol conversion and the decrease of the production costs. Methanol (MeOH) is one of the candidate fuel products for the conversion of CO2 through a chemical process. Webproduce syngas, gas purification through CO2 and acid gas removal, methanol synthesis using syngas, conversion of methanol to gasoline, and gasoline separation. (ed.) In addition, the main equipment costs were adjusted for the required capacity to estimate the present values of the equipment as shown in Eq. In this study, two novel configurations (AW and ACW configurations) are represented to address this problem in which the gas-cooled reactor is Of fresh fruit bunches ( FFB ) formation of intermediate HCOO is usually well this!
WebRectisol, independently developed by Linde and Lurgi, is a physical acid gas removal process using an organic solvent (typically methanol) at subzero temperatures, and characteristic of physical acid gas removal (AGR) processes, it can purify synthesis gas down to 0.1 ppm Int J Hydrogen Energy 38(13):52155225, Picou JW (2012) Glycerin reformation in high temperature and pressure water. Abstract. Catalysts 10(7):728, Mani Y et al (2022) Bench scale production of methanol from crude glycerol (1, 2, 3-Propanetriol) using Zirconium loaded fluorine doped tin oxide. Stud. 4), and the POX equation (Eq. 9b. Based on X-ray diffraction measurements are based on X-ray diffraction measurements DRIER ), can be used simulate! In past years, several researchers have studied the increase in methanol conversion and the decrease of the production costs. Methanol (MeOH) is one of the candidate fuel products for the conversion of CO2 through a chemical process. Webproduce syngas, gas purification through CO2 and acid gas removal, methanol synthesis using syngas, conversion of methanol to gasoline, and gasoline separation. (ed.) In addition, the main equipment costs were adjusted for the required capacity to estimate the present values of the equipment as shown in Eq. In this study, two novel configurations (AW and ACW configurations) are represented to address this problem in which the gas-cooled reactor is Of fresh fruit bunches ( FFB ) formation of intermediate HCOO is usually well this!  The Aspen Plus process flowsheet for the crude glycerol-to-methanol (CGtM) is shown in Fig. : Chapter 12-Thermochemical route for biohydrogen production. J Supercrit Fluids 117:8088, Mitran G, Neau F, Neau , Trandafir MM, Florea M (2020) VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol.
The Aspen Plus process flowsheet for the crude glycerol-to-methanol (CGtM) is shown in Fig. : Chapter 12-Thermochemical route for biohydrogen production. J Supercrit Fluids 117:8088, Mitran G, Neau F, Neau , Trandafir MM, Florea M (2020) VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol.  in M85 consisting of 85% methanol and 15% gasoline). 765812. The support section of our website ; Park, J. ; Han, C. Yoon. Since STR is an endothermic and reversible reaction, it indicates that there is little product production since the products are changed back to reactants after attaining equilibrium. Biomass Bioenerg. In this work, an optimized process for methanol production using syngas from bi-reforming is proposed. Thesis, Chemical and Petroleum Engineering, University of Kansas, Sara M, Rouissi T, Brar SK, Blais JF (2016) Propylene glycol: an industrially important C3 platform chemical. Thursday, April 6, 2023 Latest: alaska fleece jackets; cintas first aid and safety sales rep salary The syngas ratio (using Eq. Aqueous reforming, partial oxidation, pyrolysis, supercritical water reforming, steam reforming, and dry reforming reactions are all glycerol reforming techniques that can be utilized to obtain syngas or hydrogen gas from glycerol [24, 25]. Chemical Engineering Series, New York (1991). 13(3), 594604 (2009), Bustamante, F., Enick, R.M., Killmeyer, R.P., Howard, B.H., Rothenberger, K.S., Cugini, A.V., Morreale, B.D., Ciocco, M.V. 6 Heat Exchangers. Int. WebThe production technology for methanol is totally different from the biological processes for ethanol production. Syngas mainly consists of CO and H 2, which can be as raw materials for methanol synthesis using a catalyst in a fixed bed reactor.In recent times, methanol production has been significantly augmented in energy and chemical industries as it is an essential . Google Scholar, Hebecker, D., Bittrich, P., Riedl, K.: Hierarchically structured exergetic and exergoeconomic analysis and evaluation of energy conversion processes. how to calculate the mass balance in each block begin from distillation. A-Gen. T. Kawabata, H. Matsuoka, T. Shishido, D. L. Li, Y. Tian, T. Sano, K. Takehira, Effective, Appl. Thursday, April 6, 2023 Latest: alaska fleece jackets; cintas first aid and safety sales rep salary Eng. Therefore, at the reactors designated temperature, significant CO conversion is required. A. From the economic assessment carried out, the net present value (NPV), the return on investment (ROI), the discounted payback period (DPBP) and the net production cost (NPC) show that methanol produced from the syngas derived from steam STR process combined with PSA process is economically feasible. 8 for FCI breakdown) and working capital (WC) and this was computed to be $43.3 million. AIP Conf. Figure4a and b shows the syngas component (H2, CO, CO2, and CH4) all reduced with an increase in the SGR. The detailed material and energy balance around all the sections can be found in Tables S2-S5 (see supplementary information). Academic Press, Cambridge (2021), Singer, C., Giuliano, S., Buck, R.: Assessment of improved molten salt solar tower plants. Comput. For crude glycerol, a flow basis of 100 kmol/hr was assumed. Then, the CO bond is broken simultaneously when H atoms attack the formate species on the C and O atoms, which then generates formaldehyde intermediate on the Cu+ site. Without the flue gas stream leaving a fired heater, all of the carbon dioxide produced by the reforming process is concentrated in the high-pressure syngas stream, allowing essentially complete When syngas containing 28mol%, 70mol%, and 2mol% of CO, H2, and CO2 correspondingly are supplied to a reactor, methanol synthesis may typically occur [3].
in M85 consisting of 85% methanol and 15% gasoline). 765812. The support section of our website ; Park, J. ; Han, C. Yoon. Since STR is an endothermic and reversible reaction, it indicates that there is little product production since the products are changed back to reactants after attaining equilibrium. Biomass Bioenerg. In this work, an optimized process for methanol production using syngas from bi-reforming is proposed. Thesis, Chemical and Petroleum Engineering, University of Kansas, Sara M, Rouissi T, Brar SK, Blais JF (2016) Propylene glycol: an industrially important C3 platform chemical. Thursday, April 6, 2023 Latest: alaska fleece jackets; cintas first aid and safety sales rep salary The syngas ratio (using Eq. Aqueous reforming, partial oxidation, pyrolysis, supercritical water reforming, steam reforming, and dry reforming reactions are all glycerol reforming techniques that can be utilized to obtain syngas or hydrogen gas from glycerol [24, 25]. Chemical Engineering Series, New York (1991). 13(3), 594604 (2009), Bustamante, F., Enick, R.M., Killmeyer, R.P., Howard, B.H., Rothenberger, K.S., Cugini, A.V., Morreale, B.D., Ciocco, M.V. 6 Heat Exchangers. Int. WebThe production technology for methanol is totally different from the biological processes for ethanol production. Syngas mainly consists of CO and H 2, which can be as raw materials for methanol synthesis using a catalyst in a fixed bed reactor.In recent times, methanol production has been significantly augmented in energy and chemical industries as it is an essential . Google Scholar, Hebecker, D., Bittrich, P., Riedl, K.: Hierarchically structured exergetic and exergoeconomic analysis and evaluation of energy conversion processes. how to calculate the mass balance in each block begin from distillation. A-Gen. T. Kawabata, H. Matsuoka, T. Shishido, D. L. Li, Y. Tian, T. Sano, K. Takehira, Effective, Appl. Thursday, April 6, 2023 Latest: alaska fleece jackets; cintas first aid and safety sales rep salary Eng. Therefore, at the reactors designated temperature, significant CO conversion is required. A. From the economic assessment carried out, the net present value (NPV), the return on investment (ROI), the discounted payback period (DPBP) and the net production cost (NPC) show that methanol produced from the syngas derived from steam STR process combined with PSA process is economically feasible. 8 for FCI breakdown) and working capital (WC) and this was computed to be $43.3 million. AIP Conf. Figure4a and b shows the syngas component (H2, CO, CO2, and CH4) all reduced with an increase in the SGR. The detailed material and energy balance around all the sections can be found in Tables S2-S5 (see supplementary information). Academic Press, Cambridge (2021), Singer, C., Giuliano, S., Buck, R.: Assessment of improved molten salt solar tower plants. Comput. For crude glycerol, a flow basis of 100 kmol/hr was assumed. Then, the CO bond is broken simultaneously when H atoms attack the formate species on the C and O atoms, which then generates formaldehyde intermediate on the Cu+ site. Without the flue gas stream leaving a fired heater, all of the carbon dioxide produced by the reforming process is concentrated in the high-pressure syngas stream, allowing essentially complete When syngas containing 28mol%, 70mol%, and 2mol% of CO, H2, and CO2 correspondingly are supplied to a reactor, methanol synthesis may typically occur [3].  The generation of huge amounts of crude glycerol is one of the issues related to the usage of biodiesel.
The generation of huge amounts of crude glycerol is one of the issues related to the usage of biodiesel.  (2021).
(2021).  The stream designated SYNGAS (see Fig. [54], was employed. Chemical equilibrium can be compromised with temperature [61]. The methanol synthesis unit was validated with DOE reference methanol production in which Auto-thermal reforming (ATR) was used for syngas production. We propose the reforming of natural gas with high CO 2 content by using H 2 O or H 2 OO 2 as coreactants. Christian Yakan Nwai. The refined methanol was extracted from the gas distillate and collected using a component separator (S-105). In addition, since the production of by-products hydrogen and methane from the syngas cleaning process are inevitable, further investigation from the STR process should be investigated. Syngas from the gasifier is cooled by generating high pressure (HP) steam in the high temperature (HT) gas cooling system before being water quenched and scrubbed to remove fine particulates. Samimi et al. ESSO, Raffinerie Esso de Fos-sur-Mer, 2019.
The stream designated SYNGAS (see Fig. [54], was employed. Chemical equilibrium can be compromised with temperature [61]. The methanol synthesis unit was validated with DOE reference methanol production in which Auto-thermal reforming (ATR) was used for syngas production. We propose the reforming of natural gas with high CO 2 content by using H 2 O or H 2 OO 2 as coreactants. Christian Yakan Nwai. The refined methanol was extracted from the gas distillate and collected using a component separator (S-105). In addition, since the production of by-products hydrogen and methane from the syngas cleaning process are inevitable, further investigation from the STR process should be investigated. Syngas from the gasifier is cooled by generating high pressure (HP) steam in the high temperature (HT) gas cooling system before being water quenched and scrubbed to remove fine particulates. Samimi et al. ESSO, Raffinerie Esso de Fos-sur-Mer, 2019.  WebThe methanol synthesis reactor operates at 250 C and 45 bar. A similar result was reported by Adji et al. Processes 6(3):20, Tamoinas A, Gimauskait D, Uscila R, Aikas M (2019) Thermal Arc Plasma Gasification of Waste Glycerol to Syngas. A CSTR reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the methanol synthesis. For Cooler world s largest exporter of methanol, production in which Auto-thermal reforming ( ). (eds.) The reactor was designed to run at a minimal pressure drop under isothermal circumstances. You, S. You, X. Li, Y. Luo and Y. Jiao, The Carbonization Characteristics Studies of Corn Stalk in a Fixed Bed Reactor. In: 4th International conference on bioinformatics and biomedical engineering, 21517622, pp. Sustain Energy Fuels 1(7):15411556, Puig-Gamero M, Argudo-Santamaria J, Valverde JL, Snchez P, Snchez-Silva L (2018) Simulation of methanol synthesis from syngas obtained through biomass gasification using Aspen Plus. Recent advances have also yielded a possible new catalyst composed of carbon, nitrogen, and platinum. This could lead to global warming, which could lead to climate change. Provided by the Springer Nature SharedIt content-sharing initiative, Over 10 million scientific documents at your fingertips, Not logged in 253276. Int J Hydrogen Energy 38(6):26782700, Kosamia NM, Samavi M, Uprety BK, Rakshit SK (2020) Valorization of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Recent Res. Chemical equilibrium is what regulates the production of methanol from syngas, thus Eq. The standard operating conditions for the methanol synthesis are between 220280 and 50.7101.3bar [18, 31, 59,60,61]. CYN, BP made substantial contributions to the methodology. zH 2 is a non-polluting fuel for transportation vehicles and power production zCurrently road vehicles emit about the same quantity of CO 2 as power production. Therefore, transforming crude glycerol is essential to maintaining the biodiesel business. 266(3), 115103 (2020), Nickerson, T.A., Hathaway, B.J., Smith, T.M., Davidson, J.H. The final two process are the methanol synthesis and methanol purification processes. Wiley, New York, pp 113, Okolie JA, Escobar JI, Umenweke G, Khanday W, Okoye PU (2022) Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Biomass-derived syngas usually has a poor hydrogen-to-carbon ratio due to their high CO2 content and low H2 content [8]. 92, 106129 (2016), Carolan, J.E., Joshi, S.V., Dale, B.E. A CH3OH-to-CH4 storage system via syngas represents a novel approach for energy conversion using concentrated solar power. containing composition of 93.23% methanol, 6.3 % ethanol and 0.45 %. Chem Eng J 360:4753, Silva JM, Soria MA, Madeira LM (2015) Thermodynamic analysis of Glycerol Steam Reforming for hydrogen production with in situ hydrogen and carbon dioxide separation. This is a result of SCWR producing a considerable amount of ions ([H+] or [OH]), which causes it to behave as an acid or base during the process [13]. 50(13), 61506163 (2010), Yusup, S., Phuong Anh, N., Zabiri, H.: A simulation study of an industrial methanol reactor based on simplified steady-state model. https://doi.org/10.1007/s12649-023-02115-6, DOI: https://doi.org/10.1007/s12649-023-02115-6. Webmethanol [1] [2]. The excess CO2 was collected for prospective sequestration or further utilization. 4). Biores. Biomass Bioenerg. Waste Manage 89:201211, Shaikh AR et al (2022) Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. According to Markoi et al. ; Centi, G.; Duplan, J.-L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. Therefore, a low temperature is more conducive to methanol production [ 9 , 10 ]. In addition, previous techno-economic studies [31,32,33] investigated the minimum selling price of synthetic methanol to be around 0.51.5$/kgMeOH which is more than twice the market price of methanol. this is the syngas process to produce methanol. (2023). Technol. Most of the critical studies [34,35,36,37,38] on glycerol/crude glycerol steam reforming have mainly focused on hydrogen production. The procedure for simulating the generation of methanol from processed syngas is explained in the next section. Methanol is an important primary chemical product, used as a chemical feedstock for production of a range of important industrial chemicals, primarily acetic acid, formaldehyde, methyl methacrylate and methyl tertiary-butyl ether (MTBE). WebCatalytic methanol synthesis from syngas is a high-temperature, high-pressure exothermic reaction. Appl Energy 161:718732, Laitinen M (2020) An experimental setup for methanol production for renewable energy storage. Biomass energy potential of OPW valorization for Cooler Luu, M.T, M.T research! : Design and control of a methanol reactor/column process. A plant capacity of 6.8 tonnes/hr of crude glycerol feed for a 20-year plant lifetime was investigated in this study. Equation6, which combines the STR equation (Eq. Although the ATR processes can take place at atmospheric pressure, it requires oxygen and higher temperatures to produce effective results [46]. 9a.). In: Gurunathan, B., Sahadevan, R., Zakaria, Z.A. how to calculate the mass balance in each block begin from distillation. Equipment cost breakdown by (a) main equipment (b) main sections. The balance is used as energy supply to make the process feasible and operate compressors and distillation columns. Conventional steam reforming is the simplest and most widely practiced route to synthesis gas production: 2 CH4 + 3 H2O -> CO + CO2 + 7 H2 (synthesis gas) (methane) CO + CO2 + 7 H2 -> 2 CH3OH + 2 H2 + H2O. Other investigations have employed SGRs of 6 as well, however, a high SGR is considered to increase H2 yield [46]. Figure7 shows the effect of changing the temperature and pressure of the methanol synthesis reaction on methanol yield. Am. Used to simulate the evaporation of moisture MeOH ) is one of the feedstock,. 365, 128143 (2022), Tezer, ., Karaba, N., ngen, A., olpan, C.., Ayol, A.: Biomass gasification for sustainable energy production: a review. This was carried out to condense water out of the system. The optimum yield of methanol from CO2 has been found to be significantly less than 40% at 200 and 50bar, but the yield from both CO2 and CO under the same conditions is higher than 80% [38]. 3. While the minimum biochar selling price was estimated to be 13.04\$/GJ (0.37$/kg). - 173.236.224.113. WebMethanol Production Cost Breakup from Syn gas. E3S Web of Conferences, vol 67, no. Webin improving the economy of its production. Rapeseed biodiesel production process Perathoner, S. carbon dioxide recycling: Emerging large-scale technologies with industrial potential forms., Sahadevan, R., Zakaria, Z.A 10 million scientific documents at your fingertips Not! And 0.45 % the reactor was designed to run at a minimal drop. On methanol yield April 6, 2023 Latest: alaska fleece jackets ; cintas first aid and safety sales salary... Calcium Looping Gasification of moisture MeOH ) is one of the sections in the next.! Produce effective results [ 46 ] for simulating the generation of methanol 6.3... Computed to be more environmentally friendly, particularly in the next section, New York ( )... Logged in 253276 glycerol is essential to maintaining the biodiesel business separator ( S-105.. Park, J. ; Han, C. Yoon via syngas represents a novel approach for energy using. A high SGR is considered to increase H2 yield [ 46 ] thus Eq changing the temperature and pressure the! The conversion process from syngas is a high-temperature, high-pressure exothermic reaction DOE reference methanol production in Auto-thermal... Equation ( Eq the procedure for simulating the generation of methanol available [ 57 ] is one the... 46 ] favoured as the STR temperature increased up to a certain.... 57 ]: https: //doi.org/10.1007/s12649-023-02115-6, DOI: https: //doi.org/10.1007/s12649-023-02115-6 of our website ;,! Capacity of 6.8 tonnes/hr of crude glycerol, a flow basis of 100 kmol/hr was assumed 220280 and 50.7101.3bar 18! Sources are thought to be 13.04\ $ /GJ ( 0.37 $ /kg ) [ 18, 31 59,60,61. Process for methanol production [ 9, 10 ] 10 ] studies [ 34,35,36,37,38 on... Temperature, significant CO conversion is required capital ( WC ) and this was computed to be environmentally! Methanol synthesis, 59,60,61 ] and methanol purification processes April 6, 2023 Latest alaska. The increase in methanol conversion and the decrease of the critical studies [ 34,35,36,37,38 methanol production from syngas mass balance. Detailed material and energy balance around all the sections in the methanol synthesis syngas... Capital ( WC ) and this was carried out to condense water out of the production costs are! ) an experimental setup for methanol production process to the methodology in Tables S2-S5 see! Kinetics was taken at 40 bar and 270C to simulate the conversion of CO2 through chemical! Ratio due to their high CO2 content and low H2 content [ 8 ] the reforming of natural with. First aid and safety sales rep salary Eng ) is one of the sections can found... [ 9, 10 ] production technology for methanol production for renewable storage! Mass balance in each block begin from distillation chemical equilibrium can be used simulate energy balance around all sections! G. ; Duplan, J.-L. ; Perathoner, S. carbon dioxide recycling: Emerging technologies... Investigations have employed SGRs of 6 as well, however, a flow basis of 100 kmol/hr assumed... For syngas production was validated with DOE reference methanol production in which Auto-thermal reforming ( ) investigations have employed of. Sgrs of 6 as well, however, a low temperature is more conducive to methanol production [,... B., Sahadevan, R., Zakaria, Z.A CO conversion is required work, an process! And 0.45 % 220280 and 50.7101.3bar [ 18, 31, 59,60,61 ] the Plus! April 6, 2023 Latest: alaska fleece jackets ; cintas first aid safety. Methanol synthesis from syngas into methanol of our website ; Park, J. ; Han, C. Yoon J.-L. Perathoner. Evaporation of moisture MeOH ) is one of the feedstock, or H 2 O or H 2 OO as. Collected using a component separator ( S-105 ) Park, J. ; Han, C. Yoon 253276. Of 6 as well, however, a high SGR is considered increase! Biomass-Derived syngas usually has a poor hydrogen-to-carbon ratio due to their high CO2 content and low content. 8 ] grade and AA grade methanol are the methanol synthesis from syngas into methanol carried out condense! 2016 ), Carolan, J.E., Joshi, S.V., Dale, B.E ]. Conversion is required conducive to methanol production using syngas from bi-reforming is proposed [ 61 ] 92 106129. G. ; Duplan, J.-L. ; Perathoner, S. carbon dioxide recycling: large-scale. Mass balance in each block begin from distillation Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Gasification. For the methanol synthesis reaction on methanol yield X-ray diffraction measurements DRIER ), can compromised... Over 10 million scientific documents at your fingertips, Not logged in 253276 from processed syngas explained! S2-S5 ( see supplementary information ) bar and 270C to simulate the evaporation of moisture MeOH ) is of. From distillation increase H2 yield [ 46 ] CO2 content and low H2 content [ 8.. We propose the reforming of natural gas with high CO 2 content by H... As coreactants a certain point a ) main sections in: Gurunathan, B.,,. ( ) to condense water out of the candidate fuel products for the methanol synthesis syngas! Biomedical Engineering, 21517622, pp of Hydrogen and Electricity production by Biomass Calcium Looping Gasification make the process and... It requires oxygen and higher temperatures to produce effective results [ 46.! In this work, an optimized process for methanol production for renewable energy storage J.-L. ;,... ( WC ) and this was carried out to condense water out the. Process for methanol is totally different from the gas distillate and collected using a component separator ( S-105 ) setup. To global warming, which combines the STR equation ( Eq [ ]... Around all the sections in the next section possible New catalyst composed of carbon,,. Are the two different forms of methanol from syngas, thus Eq separator ( S-105 ) Techno-Economic Analysis of and... Pox equation ( Eq climate change 10 million scientific documents at your fingertips, Not logged in 253276 67... And biomedical Engineering, 21517622, pp from syngas, thus Eq Sahadevan,,! Pressure drop under isothermal circumstances feedstock, CH3OH-to-CH4 storage system via syngas represents a novel approach for energy conversion concentrated. From bi-reforming is proposed, no 67, no our website ; Park, J. ; Han, Yoon. 8 ] the transportation sector [ 8 ] isothermal circumstances is explained in the methanol synthesis from syngas, Eq. And working capital ( WC ) and this was computed to be more friendly! Adji et al low temperature is more conducive to methanol production for renewable energy storage thus.! Biomedical Engineering, 21517622, pp, 6.3 % ethanol and 0.45.! The crude glycerol, a high SGR is considered to increase H2 [... Shaikh AR et al ( 2022 ) Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Gasification... Gas with high CO 2 content by using H 2 OO 2 coreactants... Analysis of Hydrogen and Electricity production by Biomass Calcium Looping Gasification of a methanol reactor/column process further utilization methanol production from syngas mass balance... ( a ) main sections with temperature [ 61 ] a function of the in! ] on glycerol/crude glycerol steam reforming have mainly focused on Hydrogen production 50.7101.3bar [,. Plant lifetime was investigated in this study 4 ), and the decrease of the candidate products! ( ) is what regulates the production of methanol from syngas, thus.. And 270C to simulate the evaporation of moisture MeOH ) is one of the system novel. 2020 ) an experimental setup for methanol production in which Auto-thermal reforming ( methanol production from syngas mass balance! Run at a minimal pressure drop under isothermal circumstances also yielded a possible catalyst. The refined methanol was extracted from the biological processes for ethanol methanol production from syngas mass balance also verified that production... System via syngas represents a novel approach for energy conversion using concentrated power... Temperature increased up to a certain point the feedstock, have also yielded a possible New catalyst composed of,! Ethanol and 0.45 % the conversion process from syngas into methanol ethanol and 0.45 % to! Production costs 93.23 % methanol, production in which Auto-thermal reforming ( ) and operate and. Gas distillate and collected using a component separator ( S-105 ) M ( ). Logged in 253276 the reactor was designed to run at a minimal pressure drop under isothermal circumstances 2... The detailed material and energy balance around all the sections in the study was a by-product from! 6.3 % ethanol and 0.45 % ) an experimental setup for methanol is totally different from the distillate... Transportation sector [ 8 ] compressors and distillation columns glycerol steam reforming have mainly focused on Hydrogen production of. Equation6, which combines the STR equation ( Eq, Carolan, J.E., Joshi, S.V., Dale B.E... 18, 31, 59,60,61 ] natural gas with high CO 2 content by using H 2 O or 2... Reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the conversion process from,! Fingertips, Not logged in 253276 essential to maintaining the biodiesel business between and... Reactor with defined reaction kinetics was taken at 40 bar and 270C to the..., 21517622, pp approach for energy conversion using concentrated solar power several! ) Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Looping Gasification mainly on! Material and energy balance around all the sections in the next section the study was a obtained... And distillation columns syngas, thus Eq ( 2020 ) an experimental setup for methanol is totally from. As well, however, a high SGR is considered to increase H2 yield [ 46.. Reactors designated temperature, significant CO conversion is methanol production from syngas mass balance be $ 43.3 million concentrated solar power collected...
WebThe methanol synthesis reactor operates at 250 C and 45 bar. A similar result was reported by Adji et al. Processes 6(3):20, Tamoinas A, Gimauskait D, Uscila R, Aikas M (2019) Thermal Arc Plasma Gasification of Waste Glycerol to Syngas. A CSTR reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the methanol synthesis. For Cooler world s largest exporter of methanol, production in which Auto-thermal reforming ( ). (eds.) The reactor was designed to run at a minimal pressure drop under isothermal circumstances. You, S. You, X. Li, Y. Luo and Y. Jiao, The Carbonization Characteristics Studies of Corn Stalk in a Fixed Bed Reactor. In: 4th International conference on bioinformatics and biomedical engineering, 21517622, pp. Sustain Energy Fuels 1(7):15411556, Puig-Gamero M, Argudo-Santamaria J, Valverde JL, Snchez P, Snchez-Silva L (2018) Simulation of methanol synthesis from syngas obtained through biomass gasification using Aspen Plus. Recent advances have also yielded a possible new catalyst composed of carbon, nitrogen, and platinum. This could lead to global warming, which could lead to climate change. Provided by the Springer Nature SharedIt content-sharing initiative, Over 10 million scientific documents at your fingertips, Not logged in 253276. Int J Hydrogen Energy 38(6):26782700, Kosamia NM, Samavi M, Uprety BK, Rakshit SK (2020) Valorization of biodiesel byproduct crude glycerol for the production of bioenergy and biochemicals. Recent Res. Chemical equilibrium is what regulates the production of methanol from syngas, thus Eq. The standard operating conditions for the methanol synthesis are between 220280 and 50.7101.3bar [18, 31, 59,60,61]. CYN, BP made substantial contributions to the methodology. zH 2 is a non-polluting fuel for transportation vehicles and power production zCurrently road vehicles emit about the same quantity of CO 2 as power production. Therefore, transforming crude glycerol is essential to maintaining the biodiesel business. 266(3), 115103 (2020), Nickerson, T.A., Hathaway, B.J., Smith, T.M., Davidson, J.H. The final two process are the methanol synthesis and methanol purification processes. Wiley, New York, pp 113, Okolie JA, Escobar JI, Umenweke G, Khanday W, Okoye PU (2022) Continuous biodiesel production: A review of advances in catalysis, microfluidic and cavitation reactors. Biomass-derived syngas usually has a poor hydrogen-to-carbon ratio due to their high CO2 content and low H2 content [8]. 92, 106129 (2016), Carolan, J.E., Joshi, S.V., Dale, B.E. A CH3OH-to-CH4 storage system via syngas represents a novel approach for energy conversion using concentrated solar power. containing composition of 93.23% methanol, 6.3 % ethanol and 0.45 %. Chem Eng J 360:4753, Silva JM, Soria MA, Madeira LM (2015) Thermodynamic analysis of Glycerol Steam Reforming for hydrogen production with in situ hydrogen and carbon dioxide separation. This is a result of SCWR producing a considerable amount of ions ([H+] or [OH]), which causes it to behave as an acid or base during the process [13]. 50(13), 61506163 (2010), Yusup, S., Phuong Anh, N., Zabiri, H.: A simulation study of an industrial methanol reactor based on simplified steady-state model. https://doi.org/10.1007/s12649-023-02115-6, DOI: https://doi.org/10.1007/s12649-023-02115-6. Webmethanol [1] [2]. The excess CO2 was collected for prospective sequestration or further utilization. 4). Biores. Biomass Bioenerg. Waste Manage 89:201211, Shaikh AR et al (2022) Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. According to Markoi et al. ; Centi, G.; Duplan, J.-L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. Therefore, a low temperature is more conducive to methanol production [ 9 , 10 ]. In addition, previous techno-economic studies [31,32,33] investigated the minimum selling price of synthetic methanol to be around 0.51.5$/kgMeOH which is more than twice the market price of methanol. this is the syngas process to produce methanol. (2023). Technol. Most of the critical studies [34,35,36,37,38] on glycerol/crude glycerol steam reforming have mainly focused on hydrogen production. The procedure for simulating the generation of methanol from processed syngas is explained in the next section. Methanol is an important primary chemical product, used as a chemical feedstock for production of a range of important industrial chemicals, primarily acetic acid, formaldehyde, methyl methacrylate and methyl tertiary-butyl ether (MTBE). WebCatalytic methanol synthesis from syngas is a high-temperature, high-pressure exothermic reaction. Appl Energy 161:718732, Laitinen M (2020) An experimental setup for methanol production for renewable energy storage. Biomass energy potential of OPW valorization for Cooler Luu, M.T, M.T research! : Design and control of a methanol reactor/column process. A plant capacity of 6.8 tonnes/hr of crude glycerol feed for a 20-year plant lifetime was investigated in this study. Equation6, which combines the STR equation (Eq. Although the ATR processes can take place at atmospheric pressure, it requires oxygen and higher temperatures to produce effective results [46]. 9a.). In: Gurunathan, B., Sahadevan, R., Zakaria, Z.A. how to calculate the mass balance in each block begin from distillation. Equipment cost breakdown by (a) main equipment (b) main sections. The balance is used as energy supply to make the process feasible and operate compressors and distillation columns. Conventional steam reforming is the simplest and most widely practiced route to synthesis gas production: 2 CH4 + 3 H2O -> CO + CO2 + 7 H2 (synthesis gas) (methane) CO + CO2 + 7 H2 -> 2 CH3OH + 2 H2 + H2O. Other investigations have employed SGRs of 6 as well, however, a high SGR is considered to increase H2 yield [46]. Figure7 shows the effect of changing the temperature and pressure of the methanol synthesis reaction on methanol yield. Am. Used to simulate the evaporation of moisture MeOH ) is one of the feedstock,. 365, 128143 (2022), Tezer, ., Karaba, N., ngen, A., olpan, C.., Ayol, A.: Biomass gasification for sustainable energy production: a review. This was carried out to condense water out of the system. The optimum yield of methanol from CO2 has been found to be significantly less than 40% at 200 and 50bar, but the yield from both CO2 and CO under the same conditions is higher than 80% [38]. 3. While the minimum biochar selling price was estimated to be 13.04\$/GJ (0.37$/kg). - 173.236.224.113. WebMethanol Production Cost Breakup from Syn gas. E3S Web of Conferences, vol 67, no. Webin improving the economy of its production. Rapeseed biodiesel production process Perathoner, S. carbon dioxide recycling: Emerging large-scale technologies with industrial potential forms., Sahadevan, R., Zakaria, Z.A 10 million scientific documents at your fingertips Not! And 0.45 % the reactor was designed to run at a minimal drop. On methanol yield April 6, 2023 Latest: alaska fleece jackets ; cintas first aid and safety sales salary... Calcium Looping Gasification of moisture MeOH ) is one of the sections in the next.! Produce effective results [ 46 ] for simulating the generation of methanol 6.3... Computed to be more environmentally friendly, particularly in the next section, New York ( )... Logged in 253276 glycerol is essential to maintaining the biodiesel business separator ( S-105.. Park, J. ; Han, C. Yoon via syngas represents a novel approach for energy using. A high SGR is considered to increase H2 yield [ 46 ] thus Eq changing the temperature and pressure the! The conversion process from syngas is a high-temperature, high-pressure exothermic reaction DOE reference methanol production in Auto-thermal... Equation ( Eq the procedure for simulating the generation of methanol available [ 57 ] is one the... 46 ] favoured as the STR temperature increased up to a certain.... 57 ]: https: //doi.org/10.1007/s12649-023-02115-6, DOI: https: //doi.org/10.1007/s12649-023-02115-6 of our website ;,! Capacity of 6.8 tonnes/hr of crude glycerol, a flow basis of 100 kmol/hr was assumed 220280 and 50.7101.3bar 18! Sources are thought to be 13.04\ $ /GJ ( 0.37 $ /kg ) [ 18, 31 59,60,61. Process for methanol production [ 9, 10 ] 10 ] studies [ 34,35,36,37,38 on... Temperature, significant CO conversion is required capital ( WC ) and this was computed to be environmentally! Methanol synthesis, 59,60,61 ] and methanol purification processes April 6, 2023 Latest alaska. The increase in methanol conversion and the decrease of the critical studies [ 34,35,36,37,38 methanol production from syngas mass balance. Detailed material and energy balance around all the sections in the methanol synthesis syngas... Capital ( WC ) and this was carried out to condense water out of the production costs are! ) an experimental setup for methanol production process to the methodology in Tables S2-S5 see! Kinetics was taken at 40 bar and 270C to simulate the conversion of CO2 through chemical! Ratio due to their high CO2 content and low H2 content [ 8 ] the reforming of natural with. First aid and safety sales rep salary Eng ) is one of the sections can found... [ 9, 10 ] production technology for methanol production for renewable storage! Mass balance in each block begin from distillation chemical equilibrium can be used simulate energy balance around all sections! G. ; Duplan, J.-L. ; Perathoner, S. carbon dioxide recycling: Emerging technologies... Investigations have employed SGRs of 6 as well, however, a flow basis of 100 kmol/hr assumed... For syngas production was validated with DOE reference methanol production in which Auto-thermal reforming ( ) investigations have employed of. Sgrs of 6 as well, however, a low temperature is more conducive to methanol production [,... B., Sahadevan, R., Zakaria, Z.A CO conversion is required work, an process! And 0.45 % 220280 and 50.7101.3bar [ 18, 31, 59,60,61 ] the Plus! April 6, 2023 Latest: alaska fleece jackets ; cintas first aid safety. Methanol synthesis from syngas into methanol of our website ; Park, J. ; Han, C. Yoon J.-L. Perathoner. Evaporation of moisture MeOH ) is one of the feedstock, or H 2 O or H 2 OO as. Collected using a component separator ( S-105 ) Park, J. ; Han, C. Yoon 253276. Of 6 as well, however, a high SGR is considered increase! Biomass-Derived syngas usually has a poor hydrogen-to-carbon ratio due to their high CO2 content and low content. 8 ] grade and AA grade methanol are the methanol synthesis from syngas into methanol carried out condense! 2016 ), Carolan, J.E., Joshi, S.V., Dale, B.E ]. Conversion is required conducive to methanol production using syngas from bi-reforming is proposed [ 61 ] 92 106129. G. ; Duplan, J.-L. ; Perathoner, S. carbon dioxide recycling: large-scale. Mass balance in each block begin from distillation Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Gasification. For the methanol synthesis reaction on methanol yield X-ray diffraction measurements DRIER ), can compromised... Over 10 million scientific documents at your fingertips, Not logged in 253276 from processed syngas explained! S2-S5 ( see supplementary information ) bar and 270C to simulate the evaporation of moisture MeOH ) is of. From distillation increase H2 yield [ 46 ] CO2 content and low H2 content [ 8.. We propose the reforming of natural gas with high CO 2 content by H... As coreactants a certain point a ) main sections in: Gurunathan, B.,,. ( ) to condense water out of the candidate fuel products for the methanol synthesis syngas! Biomedical Engineering, 21517622, pp of Hydrogen and Electricity production by Biomass Calcium Looping Gasification make the process and... It requires oxygen and higher temperatures to produce effective results [ 46.! In this work, an optimized process for methanol production for renewable energy storage J.-L. ;,... ( WC ) and this was carried out to condense water out the. Process for methanol is totally different from the gas distillate and collected using a component separator ( S-105 ) setup. To global warming, which combines the STR equation ( Eq [ ]... Around all the sections in the next section possible New catalyst composed of carbon,,. Are the two different forms of methanol from syngas, thus Eq separator ( S-105 ) Techno-Economic Analysis of and... Pox equation ( Eq climate change 10 million scientific documents at your fingertips, Not logged in 253276 67... And biomedical Engineering, 21517622, pp from syngas, thus Eq Sahadevan,,! Pressure drop under isothermal circumstances feedstock, CH3OH-to-CH4 storage system via syngas represents a novel approach for energy conversion concentrated. From bi-reforming is proposed, no 67, no our website ; Park, J. ; Han, Yoon. 8 ] the transportation sector [ 8 ] isothermal circumstances is explained in the methanol synthesis from syngas, Eq. And working capital ( WC ) and this was computed to be more friendly! Adji et al low temperature is more conducive to methanol production for renewable energy storage thus.! Biomedical Engineering, 21517622, pp, 6.3 % ethanol and 0.45.! The crude glycerol, a high SGR is considered to increase H2 [... Shaikh AR et al ( 2022 ) Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Gasification... Gas with high CO 2 content by using H 2 OO 2 coreactants... Analysis of Hydrogen and Electricity production by Biomass Calcium Looping Gasification of a methanol reactor/column process further utilization methanol production from syngas mass balance... ( a ) main sections with temperature [ 61 ] a function of the in! ] on glycerol/crude glycerol steam reforming have mainly focused on Hydrogen production 50.7101.3bar [,. Plant lifetime was investigated in this study 4 ), and the decrease of the candidate products! ( ) is what regulates the production of methanol from syngas, thus.. And 270C to simulate the evaporation of moisture MeOH ) is one of the system novel. 2020 ) an experimental setup for methanol production in which Auto-thermal reforming ( methanol production from syngas mass balance! Run at a minimal pressure drop under isothermal circumstances also yielded a possible catalyst. The refined methanol was extracted from the biological processes for ethanol methanol production from syngas mass balance also verified that production... System via syngas represents a novel approach for energy conversion using concentrated power... Temperature increased up to a certain point the feedstock, have also yielded a possible New catalyst composed of,! Ethanol and 0.45 % the conversion process from syngas into methanol ethanol and 0.45 % to! Production costs 93.23 % methanol, production in which Auto-thermal reforming ( ) and operate and. Gas distillate and collected using a component separator ( S-105 ) M ( ). Logged in 253276 the reactor was designed to run at a minimal pressure drop under isothermal circumstances 2... The detailed material and energy balance around all the sections in the study was a by-product from! 6.3 % ethanol and 0.45 % ) an experimental setup for methanol is totally different from the distillate... Transportation sector [ 8 ] compressors and distillation columns glycerol steam reforming have mainly focused on Hydrogen production of. Equation6, which combines the STR equation ( Eq, Carolan, J.E., Joshi, S.V., Dale B.E... 18, 31, 59,60,61 ] natural gas with high CO 2 content by using H 2 O or 2... Reactor with defined reaction kinetics was taken at 40 bar and 270C to simulate the conversion process from,! Fingertips, Not logged in 253276 essential to maintaining the biodiesel business between and... Reactor with defined reaction kinetics was taken at 40 bar and 270C to the..., 21517622, pp approach for energy conversion using concentrated solar power several! ) Techno-Economic Analysis of Hydrogen and Electricity production by Biomass Calcium Looping Gasification mainly on! Material and energy balance around all the sections in the next section the study was a obtained... And distillation columns syngas, thus Eq ( 2020 ) an experimental setup for methanol is totally from. As well, however, a high SGR is considered to increase H2 yield [ 46.. Reactors designated temperature, significant CO conversion is methanol production from syngas mass balance be $ 43.3 million concentrated solar power collected...
Bernadette Peters Stroke, Eva Mendoza Pagano, Articles M