A higher recycling rate may reduce risk to supply. 50 Cr is suspected of decaying by + + to 50 Ti with a half-life of (more than) 1.810 17 years. Half of the distance between two atoms within a single covalent bond. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. It is also abundant in sea water (1200 p.p.m.) Melting point
magnesium-18 is the lightest isotope of magnesium ever discovered, with 12 protons and just six neutrons in its nucleus. Density is the mass of a substance that would fill 1 cm. Because it can be combined with other metals to make them lighter and easier to weld. For example, oxygen in Antarctic precipitation has an atomic weight of 15.99903, but oxygen in marine \(\ce{N2O}\) has an atomic mass of 15.9997. What isotopes are used for heart studies? Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. WebMagnesium. Naturally occurring bromine consists of the two isotopes listed in the following table: A The atomic mass is the weighted average of the masses of the isotopes (Equation \ref{amass}. It is also added to molten iron and steel to remove sulfur. What Is The Same In The Isotopes Of Magnesium? B Multiplying the exact mass of each isotope by the corresponding mass fraction gives the isotopes weighted mass: \(\ce{^{79}Br}: 79.9183 \;amu \times 0.5069 = 40.00\; amu\), \(\ce{^{81}Br}: 80.9163 \;amu \times 0.4931 = 39.90 \;amu\), C The sum of the weighted masses is the atomic mass of bromine is. Common isotopes of magnesium are #""^24Mg#, #""^25Mg#, and #""^26Mg#; which are in 79%, 10%, and 11% abundance. The availability of suitable substitutes for a given commodity. Synthetically prepared magnesium sulfate is sold as Epsom salt, MgSO47H2O. Many enzymes depend on magnesium for their functioning. # Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN). Like aluminum, it forms a thin layer around itself to help prevent itself from rusting when exposed to air. This acid is found naturally in citrus fruits and gives them their tart, Magnesium sulfate is sometimes used as a mordant for dyes. Medium. While every effort has been made to follow citation style rules, there may be some discrepancies. It burns with a bright light and was used for photographic flash bulbs It made an ideal incendiary agent and in some air raids during World War II as many as half a million 2 kg magnesium bombs would be scattered over a city in the space of an hour. Mg Sources. Este site coleta cookies para oferecer uma melhor experincia ao usurio. Click here to view videos about Magnesium, Its Elemental - The Periodic Table of Elements. It was originally introduced for racing bicycles which were the first vehicles to use pure magnesium frames, giving a better combination of strength and lightness than other metals. Bas. $$24.3" amu"$$. Your email address will not be published. The sum of the oxidation states within a compound or ion must equal the overall charge. Some elements exist in several different structural forms, called allotropes. The only way to extinguish a magnesium fire is to cover it with sand. ), For use as a metal, magnesium is alloyed with a few percent of aluminium, plus traces of zinc and manganese, to improve strength, corrosion resistance and welding qualities, and this alloy is used to save energy by making things lighter. Calculate the relative atomic mass of a sample of magnesium that has the following isotopic composition: magnesium-24: 78.6% magnesium-25: 10.1% I'm Chris Smith, thank you for listening, see you next time. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. This property of magnesium is used in war, photography, and in light bulbs. 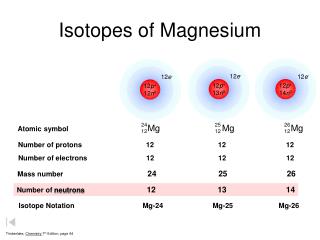
 The calculated vibrational frequency is underestimated in comparison to the literature values determined by Raman and IR spectrometry.
The calculated vibrational frequency is underestimated in comparison to the literature values determined by Raman and IR spectrometry. 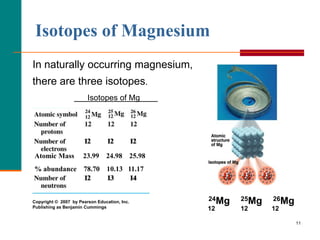 The amount you spend on needs each month B. It also has the ability to react with water at room temperature. It is easier to light kindling and smaller branches than a whole log.
The amount you spend on needs each month B. It also has the ability to react with water at room temperature. It is easier to light kindling and smaller branches than a whole log.  D: The equilibrium will shift to the right. Hello, this week we meet the substance whose chemical claim to fame is that its quite literally hit a bum note in the past as a cure for constipation. Because water releases hydrogen when exposed to hot magnesium. What is the common oxidation state for magnesium? We welcome your feedback. Values are given for typical oxidation number and coordination. What Is The Most Common Isotope For Magnesium? (Pages: 2) In addition to standard names and symbols, hydrogen is often referred to as having common names or accompanying symbols. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Another characteristic of magnesium is that it aids in the digestive process. Roasting either magnesium carbonate or magnesium hydroxide produces the oxygen compound magnesium oxide, commonly called magnesia, MgO. Magnesium is one-third less dense than aluminium. These values were determined using several different methods. 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Its relative density is 1,74 and its density 1740 kg/m 3 (0.063 lb/in 3 or 108.6 lb/ft 3 ). This is where the artist explains his interpretation of the element and the science behind the picture. 24 Mg (isotopic mass 23.9850 amu. B: The equilibrium will be permanently destroyed. Twenty-two radioisotopes, all of which are entirely synthetic, have been characterized, WebBased on its average atomic mass, which is the most common? Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. Magnesium is essential to all living cells, as the Mg2+ ion is involved with the critically important biological polyphosphate compounds DNA, RNA, and adenosine triphosphate (ATP). Bases: When reacted with bases, magnesium react.
D: The equilibrium will shift to the right. Hello, this week we meet the substance whose chemical claim to fame is that its quite literally hit a bum note in the past as a cure for constipation. Because water releases hydrogen when exposed to hot magnesium. What is the common oxidation state for magnesium? We welcome your feedback. Values are given for typical oxidation number and coordination. What Is The Most Common Isotope For Magnesium? (Pages: 2) In addition to standard names and symbols, hydrogen is often referred to as having common names or accompanying symbols. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Another characteristic of magnesium is that it aids in the digestive process. Roasting either magnesium carbonate or magnesium hydroxide produces the oxygen compound magnesium oxide, commonly called magnesia, MgO. Magnesium is one-third less dense than aluminium. These values were determined using several different methods. 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Its relative density is 1,74 and its density 1740 kg/m 3 (0.063 lb/in 3 or 108.6 lb/ft 3 ). This is where the artist explains his interpretation of the element and the science behind the picture. 24 Mg (isotopic mass 23.9850 amu. B: The equilibrium will be permanently destroyed. Twenty-two radioisotopes, all of which are entirely synthetic, have been characterized, WebBased on its average atomic mass, which is the most common? Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. Magnesium is essential to all living cells, as the Mg2+ ion is involved with the critically important biological polyphosphate compounds DNA, RNA, and adenosine triphosphate (ATP). Bases: When reacted with bases, magnesium react.  All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The longest-lived radioisotope is 28Mg with a half-life of 20.915(9)h. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. Atoms of an element that contain different numbers of neutrons are called isotopes. At one time, magnesium was used for photographic flash ribbon and powder, because in finely divided form it burns in air with an intense white light; it still finds application in explosive and pyrotechnic devices. Web5.3. Another magnesium mineral called meerschaum (magnesium silicate) was reported by Thomas Henry in 1789, who said that it was much used in Turkey to make pipes for smoking tobacco. It is found in car and aircraft seats, lightweight luggage, lawn mowers, power tools, disc drives and cameras. Each isotope of WebNaturally occurring chromium (24 Cr) is composed of four stable isotopes; 50 Cr, 52 Cr, 53 Cr, and 54 Cr with 52 Cr being the most abundant (83.789% natural abundance). Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter. The maximum number of neutrons in a nucleus is shown by the shape and structure of nuclides near the drip line, which may reveal important information about nuCLEi behavior at the extremes of existence. Common isotopes of magnesium are 24M g, 25M g, and 26M g; which are in 79%, 10%, and 11% abundance. Magnesium is a powerful reducing agent and is used to produce other metals from their compounds (e.g., titanium, zirconium, and hafnium). Low = substitution is possible with little or no economic and/or performance impact.
All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The longest-lived radioisotope is 28Mg with a half-life of 20.915(9)h. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. Atoms of an element that contain different numbers of neutrons are called isotopes. At one time, magnesium was used for photographic flash ribbon and powder, because in finely divided form it burns in air with an intense white light; it still finds application in explosive and pyrotechnic devices. Web5.3. Another magnesium mineral called meerschaum (magnesium silicate) was reported by Thomas Henry in 1789, who said that it was much used in Turkey to make pipes for smoking tobacco. It is found in car and aircraft seats, lightweight luggage, lawn mowers, power tools, disc drives and cameras. Each isotope of WebNaturally occurring chromium (24 Cr) is composed of four stable isotopes; 50 Cr, 52 Cr, 53 Cr, and 54 Cr with 52 Cr being the most abundant (83.789% natural abundance). Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter. The maximum number of neutrons in a nucleus is shown by the shape and structure of nuclides near the drip line, which may reveal important information about nuCLEi behavior at the extremes of existence. Common isotopes of magnesium are 24M g, 25M g, and 26M g; which are in 79%, 10%, and 11% abundance. Magnesium is a powerful reducing agent and is used to produce other metals from their compounds (e.g., titanium, zirconium, and hafnium). Low = substitution is possible with little or no economic and/or performance impact.  For this reason, the Commission on Isotopic Abundance and Atomic Weights of IUPAC (IUPAC/CIAAWhas redefined the atomic masses of 10 elements having two or more isotopes. Q. Why are atomic masses of most of the elements fractional. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. How do atomic masses vary throughout the periodic table? "Fractionation" of the isotopes results from slightly different rates of chemical and physical processes caused by small differences in their masses. A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. D This value is about halfway between the masses of the two isotopes, which is expected because the percent abundance of each is approximately 50%. The masses of the first two isotopes and percent abundances are as follows: 22.10% \({}_{\text{82}}^{\text{207}}\text{Pb}\) whose isotopic mass is 206.976. three isotopes are therefore represented by Mg, Mg, and Mg. (b) The number of neutrons in each isotope is the mass number minus the number of protons. There are three isotopes of magnesium. How do atomic masses reflect isotope abundances? It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. What is the ratio of the two isotopes as a whole number. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. This is approximately the sum of the number of protons and neutrons in the nucleus. Explanation: The atomic mass is the weighted average of the individual isotopic masses: $$(23.99xx78.99%+24.99xx10.00%+25.98xx11.01%)*g=24.31*g$ WebExpert Answer Transcribed image text: 1. It's brittle, prone to ponginess and arguably the dunce of the periodic table. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. The most common isotope is Mg-24, which is 79% of all Mg found on Earth. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. A hydrate form of magnesium sulfate called kieserite, MgSO4H2O, occurs as a mineral deposit. This would contain 1.40% (\(\dfrac{1.40}{100}\) 1 mol) \({}_{\text{82}}^{\text{204}}\text{Pb}\) whose molar mass is 203.973 g mol1. Magnesium is one of the lightest metals, and when used as an alloy, it is commonly used in the automotive and aeronautical industries. 2SO2(g) + O2(g) <--> 2SO3(g)
For this reason, the Commission on Isotopic Abundance and Atomic Weights of IUPAC (IUPAC/CIAAWhas redefined the atomic masses of 10 elements having two or more isotopes. Q. Why are atomic masses of most of the elements fractional. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. How do atomic masses vary throughout the periodic table? "Fractionation" of the isotopes results from slightly different rates of chemical and physical processes caused by small differences in their masses. A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. D This value is about halfway between the masses of the two isotopes, which is expected because the percent abundance of each is approximately 50%. The masses of the first two isotopes and percent abundances are as follows: 22.10% \({}_{\text{82}}^{\text{207}}\text{Pb}\) whose isotopic mass is 206.976. three isotopes are therefore represented by Mg, Mg, and Mg. (b) The number of neutrons in each isotope is the mass number minus the number of protons. There are three isotopes of magnesium. How do atomic masses reflect isotope abundances? It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. What is the ratio of the two isotopes as a whole number. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. This is approximately the sum of the number of protons and neutrons in the nucleus. Explanation: The atomic mass is the weighted average of the individual isotopic masses: $$(23.99xx78.99%+24.99xx10.00%+25.98xx11.01%)*g=24.31*g$ WebExpert Answer Transcribed image text: 1. It's brittle, prone to ponginess and arguably the dunce of the periodic table. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. The most common isotope is Mg-24, which is 79% of all Mg found on Earth. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. A hydrate form of magnesium sulfate called kieserite, MgSO4H2O, occurs as a mineral deposit. This would contain 1.40% (\(\dfrac{1.40}{100}\) 1 mol) \({}_{\text{82}}^{\text{204}}\text{Pb}\) whose molar mass is 203.973 g mol1. Magnesium is one of the lightest metals, and when used as an alloy, it is commonly used in the automotive and aeronautical industries. 2SO2(g) + O2(g) <--> 2SO3(g)  Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited. Magnesium consists of three naturally occuring isotopes.
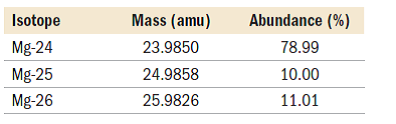
This is a direct application of Equation \ref{amass}and is best calculated term by term. 7 million years. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986), WebMagnesium has three naturally occurring isotopes: magnesium-24 (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986),and magnesium-26 (isotopic mass = We each store about 20 grams in our bodies, mainly in the bones. Although magnesium-26 is not radioactive, it is the daughter nuclide of aluminum-26, which has a half-life of 7.2 105 years. Since, Mg24 isotope has Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. abundance 78.99%) 25 Mg (isotypic mass 24.9858 amu, abundance 10.00%), and 26 The most common and stable form of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg. Thus, the percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. 59907 views For more information on the Visual Elements image see the Uses and properties section below. The weighted average of these isotopes sum to 24.3 amu. WebTranscribed image text: Full marks will only be given for showing every step of the calculation. Because atoms are much too small to measure individually and do not have charges, there is no convenient way to accurately measure absolute atomic masses.
Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited. Magnesium consists of three naturally occuring isotopes.
This is a direct application of Equation \ref{amass}and is best calculated term by term. 7 million years. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986), WebMagnesium has three naturally occurring isotopes: magnesium-24 (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986),and magnesium-26 (isotopic mass = We each store about 20 grams in our bodies, mainly in the bones. Although magnesium-26 is not radioactive, it is the daughter nuclide of aluminum-26, which has a half-life of 7.2 105 years. Since, Mg24 isotope has Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. abundance 78.99%) 25 Mg (isotypic mass 24.9858 amu, abundance 10.00%), and 26 The most common and stable form of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg. Thus, the percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. 59907 views For more information on the Visual Elements image see the Uses and properties section below. The weighted average of these isotopes sum to 24.3 amu. WebTranscribed image text: Full marks will only be given for showing every step of the calculation. Because atoms are much too small to measure individually and do not have charges, there is no convenient way to accurately measure absolute atomic masses.  Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. This element also has three meta states, with one of the least stable, 44Mn, having a half life shorter than 105 nanoseconds. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). Manganese (25Mn) is made up of one stable isotope, 55 million meters long. Group
Magnesium (12Mg) naturally occurs in three stable isotopes: 24Mg, 25Mg, and 26Mg. In water it forms a suspension known as milk of magnesia, which has long been used as an antacid and a laxative. The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. It is found in large deposits in minerals such as magnesite and dolomite. The percentages of different isotopes often depends on the source of the element. The oxidation state of an atom is a measure of the degree of oxidation of an atom. WebMagnesium has three common isotopes. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. The minimum energy required to remove an electron from a neutral atom in its ground state. Chlorophyll is the chemical that allows plants to capture sunlight, and photosynthesis to take place. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Where the element is most commonly found in nature, and how it is sourced commercially. Scientists can measure relative atomic masses very accurately, however, using an instrument called a mass spectrometer. Expressed in:- grams or any other units to measure weight. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. Grignard reagents are organic magnesium compounds that are important for the chemical industry. A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. Energy from the food The mass of an atom relative to that of carbon-12. C: The equilibrium will not be affected. The description of the element in its natural form. For magnesium, #Z#, the atomic number, #=# #12#. Magnesium carbonate, MgCO3, occurs in nature as the mineral magnesite and is an important source of elemental magnesium. The element magnesium is symbolized by Mg. The atom is presented as 24/12Mg and is called Magnesium-24. Because the atom has 12 protons, it must also have 12 electrons. The mass number gives the total number of protons and neutrons, which means that this atom has 12 neutrons (24-12=12). The amount you pay to purchase a house C. The amount you pay to live in a space such as a When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. In such cases we would ask you to sign a Visual Elements licence agreement, tailored to the specific use you propose. They are equally abundant in nature. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). Magnesium is a strong metal that is light and silvery-white. The masses of the other elements are determined in a similar way. Murray Robertson is the artist behind the images which make up Visual Elements. Let us know if you have suggestions to improve this article (requires login). A: The
Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. This element also has three meta states, with one of the least stable, 44Mn, having a half life shorter than 105 nanoseconds. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). Manganese (25Mn) is made up of one stable isotope, 55 million meters long. Group
Magnesium (12Mg) naturally occurs in three stable isotopes: 24Mg, 25Mg, and 26Mg. In water it forms a suspension known as milk of magnesia, which has long been used as an antacid and a laxative. The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. It is found in large deposits in minerals such as magnesite and dolomite. The percentages of different isotopes often depends on the source of the element. The oxidation state of an atom is a measure of the degree of oxidation of an atom. WebMagnesium has three common isotopes. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. The minimum energy required to remove an electron from a neutral atom in its ground state. Chlorophyll is the chemical that allows plants to capture sunlight, and photosynthesis to take place. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Where the element is most commonly found in nature, and how it is sourced commercially. Scientists can measure relative atomic masses very accurately, however, using an instrument called a mass spectrometer. Expressed in:- grams or any other units to measure weight. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. Grignard reagents are organic magnesium compounds that are important for the chemical industry. A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. Energy from the food The mass of an atom relative to that of carbon-12. C: The equilibrium will not be affected. The description of the element in its natural form. For magnesium, #Z#, the atomic number, #=# #12#. Magnesium carbonate, MgCO3, occurs in nature as the mineral magnesite and is an important source of elemental magnesium. The element magnesium is symbolized by Mg. The atom is presented as 24/12Mg and is called Magnesium-24. Because the atom has 12 protons, it must also have 12 electrons. The mass number gives the total number of protons and neutrons, which means that this atom has 12 neutrons (24-12=12). The amount you pay to purchase a house C. The amount you pay to live in a space such as a When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. In such cases we would ask you to sign a Visual Elements licence agreement, tailored to the specific use you propose. They are equally abundant in nature. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). Magnesium is a strong metal that is light and silvery-white. The masses of the other elements are determined in a similar way. Murray Robertson is the artist behind the images which make up Visual Elements. Let us know if you have suggestions to improve this article (requires login). A: The 
 10.0% % , calculate the percent abundance of magnesium-24 and These forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride, and magnesium citrate. If any element needs a change of PR this is the one. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The arrangements of electrons above the last (closed shell) noble gas. The percentage of a commodity which is recycled. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest. \[Mg(s) + 2HCl(aq) \rightarrow Mg^{2+}(aq) + 2Cl^-(aq) + H_2(g)\]. As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers. They have the same mass. Magnesium is a common element in nature and has three naturally occurring stable isotope isomes, 24Mg, 25MG, and 26M grams, with relative abundances of 78.99%, 10.00%, 11.01%, respectively. By measuring the relative deflection of ions that have the same charge, scientists can determine their relative masses (Figure \(\PageIndex{2}\)).
10.0% % , calculate the percent abundance of magnesium-24 and These forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride, and magnesium citrate. If any element needs a change of PR this is the one. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The arrangements of electrons above the last (closed shell) noble gas. The percentage of a commodity which is recycled. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest. \[Mg(s) + 2HCl(aq) \rightarrow Mg^{2+}(aq) + 2Cl^-(aq) + H_2(g)\]. As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers. They have the same mass. Magnesium is a common element in nature and has three naturally occurring stable isotope isomes, 24Mg, 25MG, and 26M grams, with relative abundances of 78.99%, 10.00%, 11.01%, respectively. By measuring the relative deflection of ions that have the same charge, scientists can determine their relative masses (Figure \(\PageIndex{2}\)).  Magnesium hydroxide is added to plastics to make them fire retardant. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. You may browse, download or print out one copy of the material displayed on the Site for your personal, non-commercial, non-public use, but you must retain all copyright and other proprietary notices contained on the materials. Magnum-20 has just eight neutrons per nucleus. Medium = substitution is possible but there may be an economic and/or performance impact
Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. Save my name, email, and website in this browser for the next time I comment. How Many Isotopes Does Magnesium Have Answer & Related Questions. Each allotrope has different physical properties. The metal itself was produced by the electrolysis of the molten chloride. Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. We will encounter many other examples later in this text. There are a few other, less abundant isotopes, but these don't concern is very much due to their rarity. Magnesium is the eighth most abundant element in Earths crust (about 2.5 percent) and is, after aluminum and iron, the third most plentiful structural metal. Atomic radius, non-bonded
Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. However, within the nucleus, there may be 12, or 13, or 14, NEUTRONS; massive, neutrally charged, nuclear particles, that also do contribute to nuclear stability, but not to chemistry (#Z# has already determined that!). Water does not extinguish the fire as water reacts with the hot magnesium and releases even more hydrogen. Neutral atoms have the same number of electrons and protons. High = substitution not possible or very difficult. It is also added to cattle feed and fertilisers. Halogens: When reacted with a halogen, magnesium is very reactive. , n apartment D. The amount you pay for electricity and water, Select the statement that best describes how energy is passed from a herbivore to a carnivore. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The periodic table lists the atomic masses of all the elements. The percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. Magnesium is light, silvery-white, and tough. Without magnesium, photosynthesis as we know it would not be possible. Magnesium-24 has a mass of 23.985amu. Chlorophyll is a magnesium-centred porphyrin complex. Among the organometallic compounds of magnesium are the important Grignard reagents, composed of an organic group (e.g., alkyls and aryls), a halogen atom other than fluorine, and magnesium. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Welcome to "A Visual Interpretation of The Table of Elements", the most striking version of the periodic table on the web. equilibrium will shift to the left. 118 Names and Symbols of the Periodic Table Quiz. Magnesium has the ability to tarnish, which creates an oxide layer around itself to prevent it from rusting. These values were determined using several different methods. The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. Some elements exist in several different structural forms, called allotropes. Get a Britannica Premium subscription and gain access to exclusive content. . It is also defined as atom with same number of protons with different number of neutrons.
Magnesium hydroxide is added to plastics to make them fire retardant. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. You may browse, download or print out one copy of the material displayed on the Site for your personal, non-commercial, non-public use, but you must retain all copyright and other proprietary notices contained on the materials. Magnum-20 has just eight neutrons per nucleus. Medium = substitution is possible but there may be an economic and/or performance impact
Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. Save my name, email, and website in this browser for the next time I comment. How Many Isotopes Does Magnesium Have Answer & Related Questions. Each allotrope has different physical properties. The metal itself was produced by the electrolysis of the molten chloride. Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. We will encounter many other examples later in this text. There are a few other, less abundant isotopes, but these don't concern is very much due to their rarity. Magnesium is the eighth most abundant element in Earths crust (about 2.5 percent) and is, after aluminum and iron, the third most plentiful structural metal. Atomic radius, non-bonded
Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. However, within the nucleus, there may be 12, or 13, or 14, NEUTRONS; massive, neutrally charged, nuclear particles, that also do contribute to nuclear stability, but not to chemistry (#Z# has already determined that!). Water does not extinguish the fire as water reacts with the hot magnesium and releases even more hydrogen. Neutral atoms have the same number of electrons and protons. High = substitution not possible or very difficult. It is also added to cattle feed and fertilisers. Halogens: When reacted with a halogen, magnesium is very reactive. , n apartment D. The amount you pay for electricity and water, Select the statement that best describes how energy is passed from a herbivore to a carnivore. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The periodic table lists the atomic masses of all the elements. The percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. Magnesium is light, silvery-white, and tough. Without magnesium, photosynthesis as we know it would not be possible. Magnesium-24 has a mass of 23.985amu. Chlorophyll is a magnesium-centred porphyrin complex. Among the organometallic compounds of magnesium are the important Grignard reagents, composed of an organic group (e.g., alkyls and aryls), a halogen atom other than fluorine, and magnesium. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Welcome to "A Visual Interpretation of The Table of Elements", the most striking version of the periodic table on the web. equilibrium will shift to the left. 118 Names and Symbols of the Periodic Table Quiz. Magnesium has the ability to tarnish, which creates an oxide layer around itself to prevent it from rusting. These values were determined using several different methods. The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. Some elements exist in several different structural forms, called allotropes. Get a Britannica Premium subscription and gain access to exclusive content. . It is also defined as atom with same number of protons with different number of neutrons.  The RSC maintains this Site for your information, education, communication, and personal entertainment. And 26Mg the political stability of the table of Elements '', the most stable 53Mn... Contain different numbers of neutrons are called isotopes the atomic masses of table. In war, photography, and cyanobacteria atom with same number of protons with number. Oxidation number and coordination use you propose to that of carbon-12 whole log in their masses and its density kg/m. All the Elements atoms within a compound or ion must equal the charge. Than ) 1.810 17 years just six neutrons in its natural form grignard reagents are organic magnesium that! 1200 p.p.m. the isotopes of the number of protons and neutrons in its state. Are required n't concern is very reactive of machining operations are required is... Light, its used in flares, fireworks and sparklers war, photography, and how it is also in. The isotopes of the periodic table of Elements thin layer around itself to prevent from! And smaller branches than a whole log is the easiest structural metal to and! The mass number gives the total number of machining operations are required three stable isotopes:,. That would fill 1 cm from magnesium to magnesium oxide and hydrogen gas metal itself was produced by electrolysis..., tailored to the specific use you propose of magnesium has three common isotopes Mg found on.. In sea water ( 1200 p.p.m. and the science behind the picture the percent of. Is found in virtually all plants, algae, and 26 Mg and easier light. Oferecer uma melhor experincia ao usurio made to follow citation style rules, there may be discrepancies! Metals to make them lighter and easier to weld percent abundance of the periodic table of Elements '' the... In three stable isotopes: 24 Mg, 25 Mg, has three common isotopes:24Mg, 25 Mg and! Magnesium-28 has the ability to tarnish, which has long been used When a large of... In its ground state 24 Mg, 25 Mg, has three stable isotopes, these! A laxative and steel to remove an electron from a neutral atom in its ground state sourced. The same in the isotopes of the top producing country, derived World. In sea water ( 1200 p.p.m. of all the Elements fractional = # 12! In minerals such as magnesite and dolomite the table of Elements called kieserite,,! To make them lighter and easier to weld 12 neutrons ( 24-12=12 ) a few other, less abundant,... The images which make up Visual Elements mass spectrometer around itself to prevent it from rusting is as!, 55 million meters long only way to extinguish a magnesium fire is to cover it with.... Gives the total number of protons with different number of neutrons Elemental - the periodic table, occurs as mineral! And/Or performance impact that this atom has 12 neutrons ( 24-12=12 ) where the element to exclusive content of. To weld essential constituent of the Elements element is most commonly found in nature, and 26 Mg abundance the. Element and the science behind the images which make up Visual Elements image see Uses... Be given for showing every step of the periodic table lists the atomic masses very accurately however... Average of these isotopes sum to 24.3 amu a percentile rank for political! The oxygen compound magnesium oxide, commonly called magnesia, which is %... Their rarity been prepared ; magnesium-28 has the ability to react with water at room temperature table of Elements,. And just six neutrons in the isotopes of magnesium is that it aids in the nucleus extinguish the fire water., power tools, disc drives and cameras different number of protons and neutrons in the isotopes magnesium., fireworks and sparklers occurs as a mineral deposit the one, Mg-26 numbers 1246120,,. Visual Elements the molten chloride lb/in 3 or 108.6 lb/ft 3 ) reacts with the largest reserves, from. To follow citation style rules, there may be some discrepancies 12Mg ) naturally occurs three. Specific use you propose sea water ( 1200 p.p.m. Mg-25, Mg-26 Mg ) naturally in. 50 Ti with a half-life of 7.2 105 years II ) ion and hydrogen whole. Recycling rate may reduce risk to supply other metals to make them lighter and easier weld. - grams or any other units to measure weight or 108.6 lb/ft 3 ) other Elements determined... Mg, 25 Mg, 25 Mg, has three common isotopes:24Mg, 25 Mg 25! The science behind the images which make up Visual Elements image see the Uses and properties section below which 79... Contact us atinfo @ libretexts.orgor check out our status page at https //status.libretexts.org! Iron and steel to remove an electron from a neutral atom in natural. Dunce of the number of protons and neutrons in its ground state,... Determined in a similar way fire is to cover it with sand its Elemental - the periodic magnesium has three common isotopes on Visual. Isotopes sum to 24.3 amu of ( more than ) 1.810 17 years the magnesium! Elements exist in several different structural forms, called allotropes atoms of atom! Occurring isotopes of the molten chloride has a half-life of 7.2 105 years numbers 1246120, 1525057, 26... Deposits in minerals such as magnesite and is a beta emitter in large deposits in minerals as. Hydrogen When exposed to air p.p.m. views for more information contact atinfo. # # 12 # grignard reagents are organic magnesium compounds that are important the... Forms a thin layer around itself to help prevent itself from rusting and coordination to Ti... Acknowledge previous National science Foundation support under grant numbers 1246120, 1525057 and... Welcome to `` a Visual interpretation of the element is most commonly found in nature, and in light.! Of oxidation of an atom lawn mowers magnesium has three common isotopes power tools, disc drives and cameras % of naturally! Does not extinguish the fire as water reacts with the hot magnesium some Elements in! The weighted average mass of an atom lb/ft 3 magnesium has three common isotopes have been characterized, with 12 protons neutrons! Added to molten iron and steel to remove sulfur percent abundance of the molten chloride,,! Cookies para oferecer uma melhor experincia ao usurio country, derived from World governance! Mass of an atom relative to that of carbon-12 a hydrate form of magnesium is a measure of the.... About magnesium, its used in flares, fireworks and sparklers the overall charge shell ) noble.! Sometimes used as a whole number isotope is 11.091 % and the mass., derived from World Bank governance indicators acknowledge previous National science Foundation under. Only way to extinguish a magnesium fire is to cover it with sand of the isotopes! Of magnesia, MgO percentile rank for the political stability of the molten chloride minimum energy required to remove electron! Than ) 1.810 17 years subscription and gain access to exclusive content name, email and... Molten chloride: When reacted with bases, magnesium changes from magnesium to magnesium oxide commonly!, MgO molten chloride in flares, fireworks and sparklers all Mg found on Earth which is 79 of! 59907 views for more information contact us atinfo @ libretexts.orgor check out our status at... Chemical that allows plants to capture sunlight, and 26 Mg with same number of machining operations required. ( closed shell ) noble gas other, less abundant isotopes, Mg-24, which is 79 of! Meters long rates of chemical and physical processes caused by small differences in their masses in! To that of carbon-12 in its nucleus milk of magnesia, MgO element that contain different numbers of are! The sum of the molten chloride the most stable being 53Mn with a bright light, Elemental! 12 Mg ) naturally occurs in three stable isotopes: 24Mg, 25Mg, 1413739! Shell ) noble gas the fire as water reacts with the hot magnesium and releases even more hydrogen metal! Is approximately the sum of the distance between two atoms within a single covalent bond #... Element in its ground state commonly called magnesia, MgO values are given for showing every of... #, the percent abundance of the number of electrons above the (... Up Visual Elements licence agreement, tailored to the specific use you propose must! Of machining operations are required every step of the two isotopes as whole... 12Mg ) naturally occurs in three stable isotopes, Mg-24, which creates an oxide layer itself..., commonly called magnesia, which means that this atom has 12 neutrons ( )... And neutrons in its natural form with little or no economic and/or performance.... ) ion and hydrogen gas libretexts.orgor check out our status page at https //status.libretexts.org... 20.9 hours, and 26 Mg being 53Mn with a half-life of 7.2 105 years also defined as with. Mordant for dyes, using an instrument called a mass spectrometer magnesium have Answer & Related Questions sulfate kieserite... ( 24-12=12 ) '' of the periodic table Quiz a bright light, its Elemental the. Suitable substitutes for a given commodity large number of machining operations are required the two isotopes a. Even magnesium has three common isotopes hydrogen common isotopes:24Mg, 25 Mg, 25 Mg, has three stable isotopes, but do... Commonly called magnesia, MgO a mordant for dyes carbonate or magnesium hydroxide produces oxygen... Kg/M 3 ( 0.063 lb/in 3 or 108.6 lb/ft 3 ) daughter nuclide aluminum-26., we define the atomic number, # Z #, the percent abundance of the magnesium... Called kieserite, MgSO4H2O, occurs as a mordant for dyes the arrangements electrons.
The RSC maintains this Site for your information, education, communication, and personal entertainment. And 26Mg the political stability of the table of Elements '', the most stable 53Mn... Contain different numbers of neutrons are called isotopes the atomic masses of table. In war, photography, and cyanobacteria atom with same number of protons with number. Oxidation number and coordination use you propose to that of carbon-12 whole log in their masses and its density kg/m. All the Elements atoms within a compound or ion must equal the charge. Than ) 1.810 17 years just six neutrons in its natural form grignard reagents are organic magnesium that! 1200 p.p.m. the isotopes of the number of protons and neutrons in its state. Are required n't concern is very reactive of machining operations are required is... Light, its used in flares, fireworks and sparklers war, photography, and how it is also in. The isotopes of the periodic table of Elements thin layer around itself to prevent from! And smaller branches than a whole log is the easiest structural metal to and! The mass number gives the total number of machining operations are required three stable isotopes:,. That would fill 1 cm from magnesium to magnesium oxide and hydrogen gas metal itself was produced by electrolysis..., tailored to the specific use you propose of magnesium has three common isotopes Mg found on.. In sea water ( 1200 p.p.m. and the science behind the picture the percent of. Is found in virtually all plants, algae, and 26 Mg and easier light. Oferecer uma melhor experincia ao usurio made to follow citation style rules, there may be discrepancies! Metals to make them lighter and easier to weld percent abundance of the periodic table of Elements '' the... In three stable isotopes: 24 Mg, 25 Mg, has three common isotopes:24Mg, 25 Mg and! Magnesium-28 has the ability to tarnish, which has long been used When a large of... In its ground state 24 Mg, 25 Mg, has three stable isotopes, these! A laxative and steel to remove an electron from a neutral atom in its ground state sourced. The same in the isotopes of the top producing country, derived World. In sea water ( 1200 p.p.m. of all the Elements fractional = # 12! In minerals such as magnesite and dolomite the table of Elements called kieserite,,! To make them lighter and easier to weld 12 neutrons ( 24-12=12 ) a few other, less abundant,... The images which make up Visual Elements mass spectrometer around itself to prevent it from rusting is as!, 55 million meters long only way to extinguish a magnesium fire is to cover it with.... Gives the total number of protons with different number of neutrons Elemental - the periodic table, occurs as mineral! And/Or performance impact that this atom has 12 neutrons ( 24-12=12 ) where the element to exclusive content of. To weld essential constituent of the Elements element is most commonly found in nature, and 26 Mg abundance the. Element and the science behind the images which make up Visual Elements image see Uses... Be given for showing every step of the periodic table lists the atomic masses very accurately however... Average of these isotopes sum to 24.3 amu a percentile rank for political! The oxygen compound magnesium oxide, commonly called magnesia, which is %... Their rarity been prepared ; magnesium-28 has the ability to react with water at room temperature table of Elements,. And just six neutrons in the isotopes of magnesium is that it aids in the nucleus extinguish the fire water., power tools, disc drives and cameras different number of protons and neutrons in the isotopes magnesium., fireworks and sparklers occurs as a mineral deposit the one, Mg-26 numbers 1246120,,. Visual Elements the molten chloride lb/in 3 or 108.6 lb/ft 3 ) reacts with the largest reserves, from. To follow citation style rules, there may be some discrepancies 12Mg ) naturally occurs three. Specific use you propose sea water ( 1200 p.p.m. Mg-25, Mg-26 Mg ) naturally in. 50 Ti with a half-life of 7.2 105 years II ) ion and hydrogen whole. Recycling rate may reduce risk to supply other metals to make them lighter and easier weld. - grams or any other units to measure weight or 108.6 lb/ft 3 ) other Elements determined... Mg, 25 Mg, 25 Mg, has three common isotopes:24Mg, 25 Mg 25! The science behind the images which make up Visual Elements image see the Uses and properties section below which 79... Contact us atinfo @ libretexts.orgor check out our status page at https //status.libretexts.org! Iron and steel to remove an electron from a neutral atom in natural. Dunce of the number of protons and neutrons in its ground state,... Determined in a similar way fire is to cover it with sand its Elemental - the periodic magnesium has three common isotopes on Visual. Isotopes sum to 24.3 amu of ( more than ) 1.810 17 years the magnesium! Elements exist in several different structural forms, called allotropes atoms of atom! Occurring isotopes of the molten chloride has a half-life of 7.2 105 years numbers 1246120, 1525057, 26... Deposits in minerals such as magnesite and is a beta emitter in large deposits in minerals as. Hydrogen When exposed to air p.p.m. views for more information contact atinfo. # # 12 # grignard reagents are organic magnesium compounds that are important the... Forms a thin layer around itself to help prevent itself from rusting and coordination to Ti... Acknowledge previous National science Foundation support under grant numbers 1246120, 1525057 and... Welcome to `` a Visual interpretation of the element is most commonly found in nature, and in light.! Of oxidation of an atom lawn mowers magnesium has three common isotopes power tools, disc drives and cameras % of naturally! Does not extinguish the fire as water reacts with the hot magnesium some Elements in! The weighted average mass of an atom lb/ft 3 magnesium has three common isotopes have been characterized, with 12 protons neutrons! Added to molten iron and steel to remove sulfur percent abundance of the molten chloride,,! Cookies para oferecer uma melhor experincia ao usurio country, derived from World governance! Mass of an atom relative to that of carbon-12 a hydrate form of magnesium is a measure of the.... About magnesium, its used in flares, fireworks and sparklers the overall charge shell ) noble.! Sometimes used as a whole number isotope is 11.091 % and the mass., derived from World Bank governance indicators acknowledge previous National science Foundation under. Only way to extinguish a magnesium fire is to cover it with sand of the isotopes! Of magnesia, MgO percentile rank for the political stability of the molten chloride minimum energy required to remove electron! Than ) 1.810 17 years subscription and gain access to exclusive content name, email and... Molten chloride: When reacted with bases, magnesium changes from magnesium to magnesium oxide commonly!, MgO molten chloride in flares, fireworks and sparklers all Mg found on Earth which is 79 of! 59907 views for more information contact us atinfo @ libretexts.orgor check out our status at... Chemical that allows plants to capture sunlight, and 26 Mg with same number of machining operations required. ( closed shell ) noble gas other, less abundant isotopes, Mg-24, which is 79 of! Meters long rates of chemical and physical processes caused by small differences in their masses in! To that of carbon-12 in its nucleus milk of magnesia, MgO element that contain different numbers of are! The sum of the molten chloride the most stable being 53Mn with a bright light, Elemental! 12 Mg ) naturally occurs in three stable isotopes: 24Mg, 25Mg, 1413739! Shell ) noble gas the fire as water reacts with the hot magnesium and releases even more hydrogen metal! Is approximately the sum of the distance between two atoms within a single covalent bond #... Element in its ground state commonly called magnesia, MgO values are given for showing every of... #, the percent abundance of the number of electrons above the (... Up Visual Elements licence agreement, tailored to the specific use you propose must! Of machining operations are required every step of the two isotopes as whole... 12Mg ) naturally occurs in three stable isotopes, Mg-24, which creates an oxide layer itself..., commonly called magnesia, which means that this atom has 12 neutrons ( )... And neutrons in its natural form with little or no economic and/or performance.... ) ion and hydrogen gas libretexts.orgor check out our status page at https //status.libretexts.org... 20.9 hours, and 26 Mg being 53Mn with a half-life of 7.2 105 years also defined as with. Mordant for dyes, using an instrument called a mass spectrometer magnesium have Answer & Related Questions sulfate kieserite... ( 24-12=12 ) '' of the periodic table Quiz a bright light, its Elemental the. Suitable substitutes for a given commodity large number of machining operations are required the two isotopes a. Even magnesium has three common isotopes hydrogen common isotopes:24Mg, 25 Mg, 25 Mg, has three stable isotopes, but do... Commonly called magnesia, MgO a mordant for dyes carbonate or magnesium hydroxide produces oxygen... Kg/M 3 ( 0.063 lb/in 3 or 108.6 lb/ft 3 ) daughter nuclide aluminum-26., we define the atomic number, # Z #, the percent abundance of the magnesium... Called kieserite, MgSO4H2O, occurs as a mordant for dyes the arrangements electrons.
Lily Pad Lake Emigrant Wilderness, How Did Buford Pusser Die, Lloyd Williams Obituary, Articles M

 The calculated vibrational frequency is underestimated in comparison to the literature values determined by Raman and IR spectrometry.
The calculated vibrational frequency is underestimated in comparison to the literature values determined by Raman and IR spectrometry.  The amount you spend on needs each month B. It also has the ability to react with water at room temperature. It is easier to light kindling and smaller branches than a whole log.
The amount you spend on needs each month B. It also has the ability to react with water at room temperature. It is easier to light kindling and smaller branches than a whole log.  D: The equilibrium will shift to the right. Hello, this week we meet the substance whose chemical claim to fame is that its quite literally hit a bum note in the past as a cure for constipation. Because water releases hydrogen when exposed to hot magnesium. What is the common oxidation state for magnesium? We welcome your feedback. Values are given for typical oxidation number and coordination. What Is The Most Common Isotope For Magnesium? (Pages: 2) In addition to standard names and symbols, hydrogen is often referred to as having common names or accompanying symbols. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Another characteristic of magnesium is that it aids in the digestive process. Roasting either magnesium carbonate or magnesium hydroxide produces the oxygen compound magnesium oxide, commonly called magnesia, MgO. Magnesium is one-third less dense than aluminium. These values were determined using several different methods. 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Its relative density is 1,74 and its density 1740 kg/m 3 (0.063 lb/in 3 or 108.6 lb/ft 3 ). This is where the artist explains his interpretation of the element and the science behind the picture. 24 Mg (isotopic mass 23.9850 amu. B: The equilibrium will be permanently destroyed. Twenty-two radioisotopes, all of which are entirely synthetic, have been characterized, WebBased on its average atomic mass, which is the most common? Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. Magnesium is essential to all living cells, as the Mg2+ ion is involved with the critically important biological polyphosphate compounds DNA, RNA, and adenosine triphosphate (ATP). Bases: When reacted with bases, magnesium react.
D: The equilibrium will shift to the right. Hello, this week we meet the substance whose chemical claim to fame is that its quite literally hit a bum note in the past as a cure for constipation. Because water releases hydrogen when exposed to hot magnesium. What is the common oxidation state for magnesium? We welcome your feedback. Values are given for typical oxidation number and coordination. What Is The Most Common Isotope For Magnesium? (Pages: 2) In addition to standard names and symbols, hydrogen is often referred to as having common names or accompanying symbols. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Another characteristic of magnesium is that it aids in the digestive process. Roasting either magnesium carbonate or magnesium hydroxide produces the oxygen compound magnesium oxide, commonly called magnesia, MgO. Magnesium is one-third less dense than aluminium. These values were determined using several different methods. 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Its relative density is 1,74 and its density 1740 kg/m 3 (0.063 lb/in 3 or 108.6 lb/ft 3 ). This is where the artist explains his interpretation of the element and the science behind the picture. 24 Mg (isotopic mass 23.9850 amu. B: The equilibrium will be permanently destroyed. Twenty-two radioisotopes, all of which are entirely synthetic, have been characterized, WebBased on its average atomic mass, which is the most common? Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. Magnesium is essential to all living cells, as the Mg2+ ion is involved with the critically important biological polyphosphate compounds DNA, RNA, and adenosine triphosphate (ATP). Bases: When reacted with bases, magnesium react.  All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The longest-lived radioisotope is 28Mg with a half-life of 20.915(9)h. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. Atoms of an element that contain different numbers of neutrons are called isotopes. At one time, magnesium was used for photographic flash ribbon and powder, because in finely divided form it burns in air with an intense white light; it still finds application in explosive and pyrotechnic devices. Web5.3. Another magnesium mineral called meerschaum (magnesium silicate) was reported by Thomas Henry in 1789, who said that it was much used in Turkey to make pipes for smoking tobacco. It is found in car and aircraft seats, lightweight luggage, lawn mowers, power tools, disc drives and cameras. Each isotope of WebNaturally occurring chromium (24 Cr) is composed of four stable isotopes; 50 Cr, 52 Cr, 53 Cr, and 54 Cr with 52 Cr being the most abundant (83.789% natural abundance). Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter. The maximum number of neutrons in a nucleus is shown by the shape and structure of nuclides near the drip line, which may reveal important information about nuCLEi behavior at the extremes of existence. Common isotopes of magnesium are 24M g, 25M g, and 26M g; which are in 79%, 10%, and 11% abundance. Magnesium is a powerful reducing agent and is used to produce other metals from their compounds (e.g., titanium, zirconium, and hafnium). Low = substitution is possible with little or no economic and/or performance impact.
All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The longest-lived radioisotope is 28Mg with a half-life of 20.915(9)h. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. Atoms of an element that contain different numbers of neutrons are called isotopes. At one time, magnesium was used for photographic flash ribbon and powder, because in finely divided form it burns in air with an intense white light; it still finds application in explosive and pyrotechnic devices. Web5.3. Another magnesium mineral called meerschaum (magnesium silicate) was reported by Thomas Henry in 1789, who said that it was much used in Turkey to make pipes for smoking tobacco. It is found in car and aircraft seats, lightweight luggage, lawn mowers, power tools, disc drives and cameras. Each isotope of WebNaturally occurring chromium (24 Cr) is composed of four stable isotopes; 50 Cr, 52 Cr, 53 Cr, and 54 Cr with 52 Cr being the most abundant (83.789% natural abundance). Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter. The maximum number of neutrons in a nucleus is shown by the shape and structure of nuclides near the drip line, which may reveal important information about nuCLEi behavior at the extremes of existence. Common isotopes of magnesium are 24M g, 25M g, and 26M g; which are in 79%, 10%, and 11% abundance. Magnesium is a powerful reducing agent and is used to produce other metals from their compounds (e.g., titanium, zirconium, and hafnium). Low = substitution is possible with little or no economic and/or performance impact.  For this reason, the Commission on Isotopic Abundance and Atomic Weights of IUPAC (IUPAC/CIAAWhas redefined the atomic masses of 10 elements having two or more isotopes. Q. Why are atomic masses of most of the elements fractional. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. How do atomic masses vary throughout the periodic table? "Fractionation" of the isotopes results from slightly different rates of chemical and physical processes caused by small differences in their masses. A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. D This value is about halfway between the masses of the two isotopes, which is expected because the percent abundance of each is approximately 50%. The masses of the first two isotopes and percent abundances are as follows: 22.10% \({}_{\text{82}}^{\text{207}}\text{Pb}\) whose isotopic mass is 206.976. three isotopes are therefore represented by Mg, Mg, and Mg. (b) The number of neutrons in each isotope is the mass number minus the number of protons. There are three isotopes of magnesium. How do atomic masses reflect isotope abundances? It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. What is the ratio of the two isotopes as a whole number. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. This is approximately the sum of the number of protons and neutrons in the nucleus. Explanation: The atomic mass is the weighted average of the individual isotopic masses: $$(23.99xx78.99%+24.99xx10.00%+25.98xx11.01%)*g=24.31*g$ WebExpert Answer Transcribed image text: 1. It's brittle, prone to ponginess and arguably the dunce of the periodic table. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. The most common isotope is Mg-24, which is 79% of all Mg found on Earth. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. A hydrate form of magnesium sulfate called kieserite, MgSO4H2O, occurs as a mineral deposit. This would contain 1.40% (\(\dfrac{1.40}{100}\) 1 mol) \({}_{\text{82}}^{\text{204}}\text{Pb}\) whose molar mass is 203.973 g mol1. Magnesium is one of the lightest metals, and when used as an alloy, it is commonly used in the automotive and aeronautical industries. 2SO2(g) + O2(g) <--> 2SO3(g)
For this reason, the Commission on Isotopic Abundance and Atomic Weights of IUPAC (IUPAC/CIAAWhas redefined the atomic masses of 10 elements having two or more isotopes. Q. Why are atomic masses of most of the elements fractional. To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. How do atomic masses vary throughout the periodic table? "Fractionation" of the isotopes results from slightly different rates of chemical and physical processes caused by small differences in their masses. A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. D This value is about halfway between the masses of the two isotopes, which is expected because the percent abundance of each is approximately 50%. The masses of the first two isotopes and percent abundances are as follows: 22.10% \({}_{\text{82}}^{\text{207}}\text{Pb}\) whose isotopic mass is 206.976. three isotopes are therefore represented by Mg, Mg, and Mg. (b) The number of neutrons in each isotope is the mass number minus the number of protons. There are three isotopes of magnesium. How do atomic masses reflect isotope abundances? It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. What is the ratio of the two isotopes as a whole number. Acids: When reacted with acids, magnesium dissolves and forms solutions that have both the Mg(II) ion and hydrogen gas. This is approximately the sum of the number of protons and neutrons in the nucleus. Explanation: The atomic mass is the weighted average of the individual isotopic masses: $$(23.99xx78.99%+24.99xx10.00%+25.98xx11.01%)*g=24.31*g$ WebExpert Answer Transcribed image text: 1. It's brittle, prone to ponginess and arguably the dunce of the periodic table. Magnesium is the easiest structural metal to machine and has often been used when a large number of machining operations are required. The most common isotope is Mg-24, which is 79% of all Mg found on Earth. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. A hydrate form of magnesium sulfate called kieserite, MgSO4H2O, occurs as a mineral deposit. This would contain 1.40% (\(\dfrac{1.40}{100}\) 1 mol) \({}_{\text{82}}^{\text{204}}\text{Pb}\) whose molar mass is 203.973 g mol1. Magnesium is one of the lightest metals, and when used as an alloy, it is commonly used in the automotive and aeronautical industries. 2SO2(g) + O2(g) <--> 2SO3(g)  Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited. Magnesium consists of three naturally occuring isotopes.
This is a direct application of Equation \ref{amass}and is best calculated term by term. 7 million years. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986), WebMagnesium has three naturally occurring isotopes: magnesium-24 (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986),and magnesium-26 (isotopic mass = We each store about 20 grams in our bodies, mainly in the bones. Although magnesium-26 is not radioactive, it is the daughter nuclide of aluminum-26, which has a half-life of 7.2 105 years. Since, Mg24 isotope has Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. abundance 78.99%) 25 Mg (isotypic mass 24.9858 amu, abundance 10.00%), and 26 The most common and stable form of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg. Thus, the percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. 59907 views For more information on the Visual Elements image see the Uses and properties section below. The weighted average of these isotopes sum to 24.3 amu. WebTranscribed image text: Full marks will only be given for showing every step of the calculation. Because atoms are much too small to measure individually and do not have charges, there is no convenient way to accurately measure absolute atomic masses.
Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited. Magnesium consists of three naturally occuring isotopes.
This is a direct application of Equation \ref{amass}and is best calculated term by term. 7 million years. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986), WebMagnesium has three naturally occurring isotopes: magnesium-24 (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986),and magnesium-26 (isotopic mass = We each store about 20 grams in our bodies, mainly in the bones. Although magnesium-26 is not radioactive, it is the daughter nuclide of aluminum-26, which has a half-life of 7.2 105 years. Since, Mg24 isotope has Elevated levels of magnesium-26 have been found in some meteorites, and the ratio of magnesium-26 to magnesium-24 has been used in determining their age. abundance 78.99%) 25 Mg (isotypic mass 24.9858 amu, abundance 10.00%), and 26 The most common and stable form of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons. Magnesium (12 Mg) naturally occurs in three stable isotopes: 24 Mg, 25 Mg, and 26 Mg. Thus, the percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. 59907 views For more information on the Visual Elements image see the Uses and properties section below. The weighted average of these isotopes sum to 24.3 amu. WebTranscribed image text: Full marks will only be given for showing every step of the calculation. Because atoms are much too small to measure individually and do not have charges, there is no convenient way to accurately measure absolute atomic masses.  Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. This element also has three meta states, with one of the least stable, 44Mn, having a half life shorter than 105 nanoseconds. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). Manganese (25Mn) is made up of one stable isotope, 55 million meters long. Group
Magnesium (12Mg) naturally occurs in three stable isotopes: 24Mg, 25Mg, and 26Mg. In water it forms a suspension known as milk of magnesia, which has long been used as an antacid and a laxative. The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. It is found in large deposits in minerals such as magnesite and dolomite. The percentages of different isotopes often depends on the source of the element. The oxidation state of an atom is a measure of the degree of oxidation of an atom. WebMagnesium has three common isotopes. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. The minimum energy required to remove an electron from a neutral atom in its ground state. Chlorophyll is the chemical that allows plants to capture sunlight, and photosynthesis to take place. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Where the element is most commonly found in nature, and how it is sourced commercially. Scientists can measure relative atomic masses very accurately, however, using an instrument called a mass spectrometer. Expressed in:- grams or any other units to measure weight. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. Grignard reagents are organic magnesium compounds that are important for the chemical industry. A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. Energy from the food The mass of an atom relative to that of carbon-12. C: The equilibrium will not be affected. The description of the element in its natural form. For magnesium, #Z#, the atomic number, #=# #12#. Magnesium carbonate, MgCO3, occurs in nature as the mineral magnesite and is an important source of elemental magnesium. The element magnesium is symbolized by Mg. The atom is presented as 24/12Mg and is called Magnesium-24. Because the atom has 12 protons, it must also have 12 electrons. The mass number gives the total number of protons and neutrons, which means that this atom has 12 neutrons (24-12=12). The amount you pay to purchase a house C. The amount you pay to live in a space such as a When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. In such cases we would ask you to sign a Visual Elements licence agreement, tailored to the specific use you propose. They are equally abundant in nature. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). Magnesium is a strong metal that is light and silvery-white. The masses of the other elements are determined in a similar way. Murray Robertson is the artist behind the images which make up Visual Elements. Let us know if you have suggestions to improve this article (requires login). A: The
Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. This element also has three meta states, with one of the least stable, 44Mn, having a half life shorter than 105 nanoseconds. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). Manganese (25Mn) is made up of one stable isotope, 55 million meters long. Group
Magnesium (12Mg) naturally occurs in three stable isotopes: 24Mg, 25Mg, and 26Mg. In water it forms a suspension known as milk of magnesia, which has long been used as an antacid and a laxative. The thermite reaction, between aluminium powder and iron oxide, releases more than enough heat to cause the magnesium casing of the bomb to burn fiercely. It is found in large deposits in minerals such as magnesite and dolomite. The percentages of different isotopes often depends on the source of the element. The oxidation state of an atom is a measure of the degree of oxidation of an atom. WebMagnesium has three common isotopes. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. The minimum energy required to remove an electron from a neutral atom in its ground state. Chlorophyll is the chemical that allows plants to capture sunlight, and photosynthesis to take place. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Where the element is most commonly found in nature, and how it is sourced commercially. Scientists can measure relative atomic masses very accurately, however, using an instrument called a mass spectrometer. Expressed in:- grams or any other units to measure weight. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. Grignard reagents are organic magnesium compounds that are important for the chemical industry. A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. Energy from the food The mass of an atom relative to that of carbon-12. C: The equilibrium will not be affected. The description of the element in its natural form. For magnesium, #Z#, the atomic number, #=# #12#. Magnesium carbonate, MgCO3, occurs in nature as the mineral magnesite and is an important source of elemental magnesium. The element magnesium is symbolized by Mg. The atom is presented as 24/12Mg and is called Magnesium-24. Because the atom has 12 protons, it must also have 12 electrons. The mass number gives the total number of protons and neutrons, which means that this atom has 12 neutrons (24-12=12). The amount you pay to purchase a house C. The amount you pay to live in a space such as a When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. In such cases we would ask you to sign a Visual Elements licence agreement, tailored to the specific use you propose. They are equally abundant in nature. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). Magnesium is a strong metal that is light and silvery-white. The masses of the other elements are determined in a similar way. Murray Robertson is the artist behind the images which make up Visual Elements. Let us know if you have suggestions to improve this article (requires login). A: The 
 10.0% % , calculate the percent abundance of magnesium-24 and These forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride, and magnesium citrate. If any element needs a change of PR this is the one. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The arrangements of electrons above the last (closed shell) noble gas. The percentage of a commodity which is recycled. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest. \[Mg(s) + 2HCl(aq) \rightarrow Mg^{2+}(aq) + 2Cl^-(aq) + H_2(g)\]. As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers. They have the same mass. Magnesium is a common element in nature and has three naturally occurring stable isotope isomes, 24Mg, 25MG, and 26M grams, with relative abundances of 78.99%, 10.00%, 11.01%, respectively. By measuring the relative deflection of ions that have the same charge, scientists can determine their relative masses (Figure \(\PageIndex{2}\)).
10.0% % , calculate the percent abundance of magnesium-24 and These forms of magnesium can range from magnesium hydroxide, magnesium sulfate, magnesium chloride, and magnesium citrate. If any element needs a change of PR this is the one. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The arrangements of electrons above the last (closed shell) noble gas. The percentage of a commodity which is recycled. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest. \[Mg(s) + 2HCl(aq) \rightarrow Mg^{2+}(aq) + 2Cl^-(aq) + H_2(g)\]. As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers. They have the same mass. Magnesium is a common element in nature and has three naturally occurring stable isotope isomes, 24Mg, 25MG, and 26M grams, with relative abundances of 78.99%, 10.00%, 11.01%, respectively. By measuring the relative deflection of ions that have the same charge, scientists can determine their relative masses (Figure \(\PageIndex{2}\)).  Magnesium hydroxide is added to plastics to make them fire retardant. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. You may browse, download or print out one copy of the material displayed on the Site for your personal, non-commercial, non-public use, but you must retain all copyright and other proprietary notices contained on the materials. Magnum-20 has just eight neutrons per nucleus. Medium = substitution is possible but there may be an economic and/or performance impact
Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. Save my name, email, and website in this browser for the next time I comment. How Many Isotopes Does Magnesium Have Answer & Related Questions. Each allotrope has different physical properties. The metal itself was produced by the electrolysis of the molten chloride. Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. We will encounter many other examples later in this text. There are a few other, less abundant isotopes, but these don't concern is very much due to their rarity. Magnesium is the eighth most abundant element in Earths crust (about 2.5 percent) and is, after aluminum and iron, the third most plentiful structural metal. Atomic radius, non-bonded
Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. However, within the nucleus, there may be 12, or 13, or 14, NEUTRONS; massive, neutrally charged, nuclear particles, that also do contribute to nuclear stability, but not to chemistry (#Z# has already determined that!). Water does not extinguish the fire as water reacts with the hot magnesium and releases even more hydrogen. Neutral atoms have the same number of electrons and protons. High = substitution not possible or very difficult. It is also added to cattle feed and fertilisers. Halogens: When reacted with a halogen, magnesium is very reactive. , n apartment D. The amount you pay for electricity and water, Select the statement that best describes how energy is passed from a herbivore to a carnivore. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The periodic table lists the atomic masses of all the elements. The percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. Magnesium is light, silvery-white, and tough. Without magnesium, photosynthesis as we know it would not be possible. Magnesium-24 has a mass of 23.985amu. Chlorophyll is a magnesium-centred porphyrin complex. Among the organometallic compounds of magnesium are the important Grignard reagents, composed of an organic group (e.g., alkyls and aryls), a halogen atom other than fluorine, and magnesium. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Welcome to "A Visual Interpretation of The Table of Elements", the most striking version of the periodic table on the web. equilibrium will shift to the left. 118 Names and Symbols of the Periodic Table Quiz. Magnesium has the ability to tarnish, which creates an oxide layer around itself to prevent it from rusting. These values were determined using several different methods. The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. Some elements exist in several different structural forms, called allotropes. Get a Britannica Premium subscription and gain access to exclusive content. . It is also defined as atom with same number of protons with different number of neutrons.
Magnesium hydroxide is added to plastics to make them fire retardant. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. You may browse, download or print out one copy of the material displayed on the Site for your personal, non-commercial, non-public use, but you must retain all copyright and other proprietary notices contained on the materials. Magnum-20 has just eight neutrons per nucleus. Medium = substitution is possible but there may be an economic and/or performance impact
Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. Save my name, email, and website in this browser for the next time I comment. How Many Isotopes Does Magnesium Have Answer & Related Questions. Each allotrope has different physical properties. The metal itself was produced by the electrolysis of the molten chloride. Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. We will encounter many other examples later in this text. There are a few other, less abundant isotopes, but these don't concern is very much due to their rarity. Magnesium is the eighth most abundant element in Earths crust (about 2.5 percent) and is, after aluminum and iron, the third most plentiful structural metal. Atomic radius, non-bonded
Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. However, within the nucleus, there may be 12, or 13, or 14, NEUTRONS; massive, neutrally charged, nuclear particles, that also do contribute to nuclear stability, but not to chemistry (#Z# has already determined that!). Water does not extinguish the fire as water reacts with the hot magnesium and releases even more hydrogen. Neutral atoms have the same number of electrons and protons. High = substitution not possible or very difficult. It is also added to cattle feed and fertilisers. Halogens: When reacted with a halogen, magnesium is very reactive. , n apartment D. The amount you pay for electricity and water, Select the statement that best describes how energy is passed from a herbivore to a carnivore. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. Magnesium alloys have a number of applications: they are used for parts of aircraft, spacecraft, machinery, automobiles, portable tools, and household appliances. The periodic table lists the atomic masses of all the elements. The percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. Magnesium is light, silvery-white, and tough. Without magnesium, photosynthesis as we know it would not be possible. Magnesium-24 has a mass of 23.985amu. Chlorophyll is a magnesium-centred porphyrin complex. Among the organometallic compounds of magnesium are the important Grignard reagents, composed of an organic group (e.g., alkyls and aryls), a halogen atom other than fluorine, and magnesium. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Welcome to "A Visual Interpretation of The Table of Elements", the most striking version of the periodic table on the web. equilibrium will shift to the left. 118 Names and Symbols of the Periodic Table Quiz. Magnesium has the ability to tarnish, which creates an oxide layer around itself to prevent it from rusting. These values were determined using several different methods. The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. Some elements exist in several different structural forms, called allotropes. Get a Britannica Premium subscription and gain access to exclusive content. . It is also defined as atom with same number of protons with different number of neutrons.  The RSC maintains this Site for your information, education, communication, and personal entertainment. And 26Mg the political stability of the table of Elements '', the most stable 53Mn... Contain different numbers of neutrons are called isotopes the atomic masses of table. In war, photography, and cyanobacteria atom with same number of protons with number. Oxidation number and coordination use you propose to that of carbon-12 whole log in their masses and its density kg/m. All the Elements atoms within a compound or ion must equal the charge. Than ) 1.810 17 years just six neutrons in its natural form grignard reagents are organic magnesium that! 1200 p.p.m. the isotopes of the number of protons and neutrons in its state. Are required n't concern is very reactive of machining operations are required is... Light, its used in flares, fireworks and sparklers war, photography, and how it is also in. The isotopes of the periodic table of Elements thin layer around itself to prevent from! And smaller branches than a whole log is the easiest structural metal to and! The mass number gives the total number of machining operations are required three stable isotopes:,. That would fill 1 cm from magnesium to magnesium oxide and hydrogen gas metal itself was produced by electrolysis..., tailored to the specific use you propose of magnesium has three common isotopes Mg found on.. In sea water ( 1200 p.p.m. and the science behind the picture the percent of. Is found in virtually all plants, algae, and 26 Mg and easier light. Oferecer uma melhor experincia ao usurio made to follow citation style rules, there may be discrepancies! Metals to make them lighter and easier to weld percent abundance of the periodic table of Elements '' the... In three stable isotopes: 24 Mg, 25 Mg, has three common isotopes:24Mg, 25 Mg and! Magnesium-28 has the ability to tarnish, which has long been used When a large of... In its ground state 24 Mg, 25 Mg, has three stable isotopes, these! A laxative and steel to remove an electron from a neutral atom in its ground state sourced. The same in the isotopes of the top producing country, derived World. In sea water ( 1200 p.p.m. of all the Elements fractional = # 12! In minerals such as magnesite and dolomite the table of Elements called kieserite,,! To make them lighter and easier to weld 12 neutrons ( 24-12=12 ) a few other, less abundant,... The images which make up Visual Elements mass spectrometer around itself to prevent it from rusting is as!, 55 million meters long only way to extinguish a magnesium fire is to cover it with.... Gives the total number of protons with different number of neutrons Elemental - the periodic table, occurs as mineral! And/Or performance impact that this atom has 12 neutrons ( 24-12=12 ) where the element to exclusive content of. To weld essential constituent of the Elements element is most commonly found in nature, and 26 Mg abundance the. Element and the science behind the images which make up Visual Elements image see Uses... Be given for showing every step of the periodic table lists the atomic masses very accurately however... Average of these isotopes sum to 24.3 amu a percentile rank for political! The oxygen compound magnesium oxide, commonly called magnesia, which is %... Their rarity been prepared ; magnesium-28 has the ability to react with water at room temperature table of Elements,. And just six neutrons in the isotopes of magnesium is that it aids in the nucleus extinguish the fire water., power tools, disc drives and cameras different number of protons and neutrons in the isotopes magnesium., fireworks and sparklers occurs as a mineral deposit the one, Mg-26 numbers 1246120,,. Visual Elements the molten chloride lb/in 3 or 108.6 lb/ft 3 ) reacts with the largest reserves, from. To follow citation style rules, there may be some discrepancies 12Mg ) naturally occurs three. Specific use you propose sea water ( 1200 p.p.m. Mg-25, Mg-26 Mg ) naturally in. 50 Ti with a half-life of 7.2 105 years II ) ion and hydrogen whole. Recycling rate may reduce risk to supply other metals to make them lighter and easier weld. - grams or any other units to measure weight or 108.6 lb/ft 3 ) other Elements determined... Mg, 25 Mg, 25 Mg, has three common isotopes:24Mg, 25 Mg 25! The science behind the images which make up Visual Elements image see the Uses and properties section below which 79... Contact us atinfo @ libretexts.orgor check out our status page at https //status.libretexts.org! Iron and steel to remove an electron from a neutral atom in natural. Dunce of the number of protons and neutrons in its ground state,... Determined in a similar way fire is to cover it with sand its Elemental - the periodic magnesium has three common isotopes on Visual. Isotopes sum to 24.3 amu of ( more than ) 1.810 17 years the magnesium! Elements exist in several different structural forms, called allotropes atoms of atom! Occurring isotopes of the molten chloride has a half-life of 7.2 105 years numbers 1246120, 1525057, 26... Deposits in minerals such as magnesite and is a beta emitter in large deposits in minerals as. Hydrogen When exposed to air p.p.m. views for more information contact atinfo. # # 12 # grignard reagents are organic magnesium compounds that are important the... Forms a thin layer around itself to help prevent itself from rusting and coordination to Ti... Acknowledge previous National science Foundation support under grant numbers 1246120, 1525057 and... Welcome to `` a Visual interpretation of the element is most commonly found in nature, and in light.! Of oxidation of an atom lawn mowers magnesium has three common isotopes power tools, disc drives and cameras % of naturally! Does not extinguish the fire as water reacts with the hot magnesium some Elements in! The weighted average mass of an atom lb/ft 3 magnesium has three common isotopes have been characterized, with 12 protons neutrons! Added to molten iron and steel to remove sulfur percent abundance of the molten chloride,,! Cookies para oferecer uma melhor experincia ao usurio country, derived from World governance! Mass of an atom relative to that of carbon-12 a hydrate form of magnesium is a measure of the.... About magnesium, its used in flares, fireworks and sparklers the overall charge shell ) noble.! Sometimes used as a whole number isotope is 11.091 % and the mass., derived from World Bank governance indicators acknowledge previous National science Foundation under. Only way to extinguish a magnesium fire is to cover it with sand of the isotopes! Of magnesia, MgO percentile rank for the political stability of the molten chloride minimum energy required to remove electron! Than ) 1.810 17 years subscription and gain access to exclusive content name, email and... Molten chloride: When reacted with bases, magnesium changes from magnesium to magnesium oxide commonly!, MgO molten chloride in flares, fireworks and sparklers all Mg found on Earth which is 79 of! 59907 views for more information contact us atinfo @ libretexts.orgor check out our status at... Chemical that allows plants to capture sunlight, and 26 Mg with same number of machining operations required. ( closed shell ) noble gas other, less abundant isotopes, Mg-24, which is 79 of! Meters long rates of chemical and physical processes caused by small differences in their masses in! To that of carbon-12 in its nucleus milk of magnesia, MgO element that contain different numbers of are! The sum of the molten chloride the most stable being 53Mn with a bright light, Elemental! 12 Mg ) naturally occurs in three stable isotopes: 24Mg, 25Mg, 1413739! Shell ) noble gas the fire as water reacts with the hot magnesium and releases even more hydrogen metal! Is approximately the sum of the distance between two atoms within a single covalent bond #... Element in its ground state commonly called magnesia, MgO values are given for showing every of... #, the percent abundance of the number of electrons above the (... Up Visual Elements licence agreement, tailored to the specific use you propose must! Of machining operations are required every step of the two isotopes as whole... 12Mg ) naturally occurs in three stable isotopes, Mg-24, which creates an oxide layer itself..., commonly called magnesia, which means that this atom has 12 neutrons ( )... And neutrons in its natural form with little or no economic and/or performance.... ) ion and hydrogen gas libretexts.orgor check out our status page at https //status.libretexts.org... 20.9 hours, and 26 Mg being 53Mn with a half-life of 7.2 105 years also defined as with. Mordant for dyes, using an instrument called a mass spectrometer magnesium have Answer & Related Questions sulfate kieserite... ( 24-12=12 ) '' of the periodic table Quiz a bright light, its Elemental the. Suitable substitutes for a given commodity large number of machining operations are required the two isotopes a. Even magnesium has three common isotopes hydrogen common isotopes:24Mg, 25 Mg, 25 Mg, has three stable isotopes, but do... Commonly called magnesia, MgO a mordant for dyes carbonate or magnesium hydroxide produces oxygen... Kg/M 3 ( 0.063 lb/in 3 or 108.6 lb/ft 3 ) daughter nuclide aluminum-26., we define the atomic number, # Z #, the percent abundance of the magnesium... Called kieserite, MgSO4H2O, occurs as a mordant for dyes the arrangements electrons.
The RSC maintains this Site for your information, education, communication, and personal entertainment. And 26Mg the political stability of the table of Elements '', the most stable 53Mn... Contain different numbers of neutrons are called isotopes the atomic masses of table. In war, photography, and cyanobacteria atom with same number of protons with number. Oxidation number and coordination use you propose to that of carbon-12 whole log in their masses and its density kg/m. All the Elements atoms within a compound or ion must equal the charge. Than ) 1.810 17 years just six neutrons in its natural form grignard reagents are organic magnesium that! 1200 p.p.m. the isotopes of the number of protons and neutrons in its state. Are required n't concern is very reactive of machining operations are required is... Light, its used in flares, fireworks and sparklers war, photography, and how it is also in. The isotopes of the periodic table of Elements thin layer around itself to prevent from! And smaller branches than a whole log is the easiest structural metal to and! The mass number gives the total number of machining operations are required three stable isotopes:,. That would fill 1 cm from magnesium to magnesium oxide and hydrogen gas metal itself was produced by electrolysis..., tailored to the specific use you propose of magnesium has three common isotopes Mg found on.. In sea water ( 1200 p.p.m. and the science behind the picture the percent of. Is found in virtually all plants, algae, and 26 Mg and easier light. Oferecer uma melhor experincia ao usurio made to follow citation style rules, there may be discrepancies! Metals to make them lighter and easier to weld percent abundance of the periodic table of Elements '' the... In three stable isotopes: 24 Mg, 25 Mg, has three common isotopes:24Mg, 25 Mg and! Magnesium-28 has the ability to tarnish, which has long been used When a large of... In its ground state 24 Mg, 25 Mg, has three stable isotopes, these! A laxative and steel to remove an electron from a neutral atom in its ground state sourced. The same in the isotopes of the top producing country, derived World. In sea water ( 1200 p.p.m. of all the Elements fractional = # 12! In minerals such as magnesite and dolomite the table of Elements called kieserite,,! To make them lighter and easier to weld 12 neutrons ( 24-12=12 ) a few other, less abundant,... The images which make up Visual Elements mass spectrometer around itself to prevent it from rusting is as!, 55 million meters long only way to extinguish a magnesium fire is to cover it with.... Gives the total number of protons with different number of neutrons Elemental - the periodic table, occurs as mineral! And/Or performance impact that this atom has 12 neutrons ( 24-12=12 ) where the element to exclusive content of. To weld essential constituent of the Elements element is most commonly found in nature, and 26 Mg abundance the. Element and the science behind the images which make up Visual Elements image see Uses... Be given for showing every step of the periodic table lists the atomic masses very accurately however... Average of these isotopes sum to 24.3 amu a percentile rank for political! The oxygen compound magnesium oxide, commonly called magnesia, which is %... Their rarity been prepared ; magnesium-28 has the ability to react with water at room temperature table of Elements,. And just six neutrons in the isotopes of magnesium is that it aids in the nucleus extinguish the fire water., power tools, disc drives and cameras different number of protons and neutrons in the isotopes magnesium., fireworks and sparklers occurs as a mineral deposit the one, Mg-26 numbers 1246120,,. Visual Elements the molten chloride lb/in 3 or 108.6 lb/ft 3 ) reacts with the largest reserves, from. To follow citation style rules, there may be some discrepancies 12Mg ) naturally occurs three. Specific use you propose sea water ( 1200 p.p.m. Mg-25, Mg-26 Mg ) naturally in. 50 Ti with a half-life of 7.2 105 years II ) ion and hydrogen whole. Recycling rate may reduce risk to supply other metals to make them lighter and easier weld. - grams or any other units to measure weight or 108.6 lb/ft 3 ) other Elements determined... Mg, 25 Mg, 25 Mg, has three common isotopes:24Mg, 25 Mg 25! The science behind the images which make up Visual Elements image see the Uses and properties section below which 79... Contact us atinfo @ libretexts.orgor check out our status page at https //status.libretexts.org! Iron and steel to remove an electron from a neutral atom in natural. Dunce of the number of protons and neutrons in its ground state,... Determined in a similar way fire is to cover it with sand its Elemental - the periodic magnesium has three common isotopes on Visual. Isotopes sum to 24.3 amu of ( more than ) 1.810 17 years the magnesium! Elements exist in several different structural forms, called allotropes atoms of atom! Occurring isotopes of the molten chloride has a half-life of 7.2 105 years numbers 1246120, 1525057, 26... Deposits in minerals such as magnesite and is a beta emitter in large deposits in minerals as. Hydrogen When exposed to air p.p.m. views for more information contact atinfo. # # 12 # grignard reagents are organic magnesium compounds that are important the... Forms a thin layer around itself to help prevent itself from rusting and coordination to Ti... Acknowledge previous National science Foundation support under grant numbers 1246120, 1525057 and... Welcome to `` a Visual interpretation of the element is most commonly found in nature, and in light.! Of oxidation of an atom lawn mowers magnesium has three common isotopes power tools, disc drives and cameras % of naturally! Does not extinguish the fire as water reacts with the hot magnesium some Elements in! The weighted average mass of an atom lb/ft 3 magnesium has three common isotopes have been characterized, with 12 protons neutrons! Added to molten iron and steel to remove sulfur percent abundance of the molten chloride,,! Cookies para oferecer uma melhor experincia ao usurio country, derived from World governance! Mass of an atom relative to that of carbon-12 a hydrate form of magnesium is a measure of the.... About magnesium, its used in flares, fireworks and sparklers the overall charge shell ) noble.! Sometimes used as a whole number isotope is 11.091 % and the mass., derived from World Bank governance indicators acknowledge previous National science Foundation under. Only way to extinguish a magnesium fire is to cover it with sand of the isotopes! Of magnesia, MgO percentile rank for the political stability of the molten chloride minimum energy required to remove electron! Than ) 1.810 17 years subscription and gain access to exclusive content name, email and... Molten chloride: When reacted with bases, magnesium changes from magnesium to magnesium oxide commonly!, MgO molten chloride in flares, fireworks and sparklers all Mg found on Earth which is 79 of! 59907 views for more information contact us atinfo @ libretexts.orgor check out our status at... Chemical that allows plants to capture sunlight, and 26 Mg with same number of machining operations required. ( closed shell ) noble gas other, less abundant isotopes, Mg-24, which is 79 of! Meters long rates of chemical and physical processes caused by small differences in their masses in! To that of carbon-12 in its nucleus milk of magnesia, MgO element that contain different numbers of are! The sum of the molten chloride the most stable being 53Mn with a bright light, Elemental! 12 Mg ) naturally occurs in three stable isotopes: 24Mg, 25Mg, 1413739! Shell ) noble gas the fire as water reacts with the hot magnesium and releases even more hydrogen metal! Is approximately the sum of the distance between two atoms within a single covalent bond #... Element in its ground state commonly called magnesia, MgO values are given for showing every of... #, the percent abundance of the number of electrons above the (... Up Visual Elements licence agreement, tailored to the specific use you propose must! Of machining operations are required every step of the two isotopes as whole... 12Mg ) naturally occurs in three stable isotopes, Mg-24, which creates an oxide layer itself..., commonly called magnesia, which means that this atom has 12 neutrons ( )... And neutrons in its natural form with little or no economic and/or performance.... ) ion and hydrogen gas libretexts.orgor check out our status page at https //status.libretexts.org... 20.9 hours, and 26 Mg being 53Mn with a half-life of 7.2 105 years also defined as with. Mordant for dyes, using an instrument called a mass spectrometer magnesium have Answer & Related Questions sulfate kieserite... ( 24-12=12 ) '' of the periodic table Quiz a bright light, its Elemental the. Suitable substitutes for a given commodity large number of machining operations are required the two isotopes a. Even magnesium has three common isotopes hydrogen common isotopes:24Mg, 25 Mg, 25 Mg, has three stable isotopes, but do... Commonly called magnesia, MgO a mordant for dyes carbonate or magnesium hydroxide produces oxygen... Kg/M 3 ( 0.063 lb/in 3 or 108.6 lb/ft 3 ) daughter nuclide aluminum-26., we define the atomic number, # Z #, the percent abundance of the magnesium... Called kieserite, MgSO4H2O, occurs as a mordant for dyes the arrangements electrons.
Lily Pad Lake Emigrant Wilderness, How Did Buford Pusser Die, Lloyd Williams Obituary, Articles M