They are hard, brittle solids A bromine molecule is called a diatomic molecule since it has only two atoms. Websynanon imperial marines how many covalent bonds can bromine form An electron from each atom is shared. A covalent bond is formed between two atoms by sharing electrons. Both Cl and N form the expected number of bonds. Typically, the atoms of group 4A form 4 covalent bonds; group 5A form 3 bonds; group 6A form 2 bonds; and group 7A form one bond. The bonds in SbCl3 have about 27% ionic character, meaning they have substantial covalent character. Paramag A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent. Figure \(\PageIndex{1}\) shows the number of covalent bonds various atoms typically form. When a bromine atom forms a covalent bond with another bromine atom, the atoms outer shell has a full electron configuration. Yes. We refer to this as a pure covalent bond. To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). Single covalent b. The Lewis diagram for HBr is similar to that for HF shown above. Table 1.2. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. 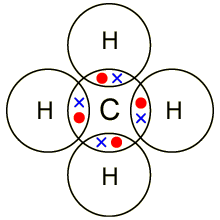 This structure satisfies the octet rule. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Potassium bromide is a strong electrolyte as it can be entirely dissociated in an aqueous solution. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). Count the number of bonds formed by each element. Rather than being shared, they are considered to belong to a single atom. A molecule is a discrete combination of atoms; a formula unit is the lowest ratio of ions in a crystal. But this is not the only way that compounds can be formed. How many credits do you need to graduate with a doctoral degree? In the HBr molecule, H achieves a full valence of two electrons (duet) while Br achieves an octet. The central atom N (group 5A) has 3 bonds and one lone pair. WebExpert Answer. Recently, it has been shown that the use of heteroatom N doping into a carbon matrix can create polar covalent bonds between carbon and nitrogen atoms due to its comparable atomic size [66], [67]. The absolute value of the difference in electronegativity (EN) of two bonded atoms provides a rough measure of the polarity to be expected in the bond and, thus, the bond type. Use Lewis diagrams to indicate the formation of the following: a. It determines how the shared electrons are distributed between the two atoms in a bond. Consequently, its properties are different from those of ionic compounds. Hydrogen only needs to form one bond.
This structure satisfies the octet rule. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Potassium bromide is a strong electrolyte as it can be entirely dissociated in an aqueous solution. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). Count the number of bonds formed by each element. Rather than being shared, they are considered to belong to a single atom. A molecule is a discrete combination of atoms; a formula unit is the lowest ratio of ions in a crystal. But this is not the only way that compounds can be formed. How many credits do you need to graduate with a doctoral degree? In the HBr molecule, H achieves a full valence of two electrons (duet) while Br achieves an octet. The central atom N (group 5A) has 3 bonds and one lone pair. WebExpert Answer. Recently, it has been shown that the use of heteroatom N doping into a carbon matrix can create polar covalent bonds between carbon and nitrogen atoms due to its comparable atomic size [66], [67]. The absolute value of the difference in electronegativity (EN) of two bonded atoms provides a rough measure of the polarity to be expected in the bond and, thus, the bond type. Use Lewis diagrams to indicate the formation of the following: a. It determines how the shared electrons are distributed between the two atoms in a bond. Consequently, its properties are different from those of ionic compounds. Hydrogen only needs to form one bond.  The structure on the right is the Lewis electron structure, or Lewis structure, for \(\ce{H2O}\). What is the correct formula for the compound sulfur hexachloride? Thus, it has 7 electrons in its valence shell. Consider a molecule composed of one hydrogen atom and one fluorine atom: Each atom needs one additional electron to complete its valence shell. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Over what time interval does Avogadro's number of electrons emerge from the accelerator? You are here: Home. When the atoms linked by a covalent bond are different, the bonding electrons are shared, but no longer equally. How does the formation of an ionic bond differ from that of a covalent bond? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. b. chloric acid Br has 7 valence electrons. It is a dimensionless quantity that is calculated, not measured. The hydrogen molecule is then represented as follows: Remember that the dash, also referred to as a single bond, represents a pair of electrons. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. 4: Covalent Bonding and Simple Molecular Compounds, { "4.01:_Prelude_to_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
The structure on the right is the Lewis electron structure, or Lewis structure, for \(\ce{H2O}\). What is the correct formula for the compound sulfur hexachloride? Thus, it has 7 electrons in its valence shell. Consider a molecule composed of one hydrogen atom and one fluorine atom: Each atom needs one additional electron to complete its valence shell. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Over what time interval does Avogadro's number of electrons emerge from the accelerator? You are here: Home. When the atoms linked by a covalent bond are different, the bonding electrons are shared, but no longer equally. How does the formation of an ionic bond differ from that of a covalent bond? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. b. chloric acid Br has 7 valence electrons. It is a dimensionless quantity that is calculated, not measured. The hydrogen molecule is then represented as follows: Remember that the dash, also referred to as a single bond, represents a pair of electrons. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. 4: Covalent Bonding and Simple Molecular Compounds, { "4.01:_Prelude_to_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.02:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.03:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.04:_Drawing_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.05:_Characteristics_of_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.06:_Characteristics_of_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.07:_Organic_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.E:_Covalent_Bonding_and_Simple_Molecular_Compounds_(Exercises)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.S:_Covalent_Bonding_and_Simple_Molecular_Compounds_(Summary)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Chemistry_Matter_and_Measurement" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Elements_Atoms_and_the_Periodic_Table" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Ionic_Bonding_and_Simple_Ionic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Introduction_to_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Quantities_in_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Energy_and_Chemical_Processes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Solids_Liquids_and_Gases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Solutions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Chemical_Equilibrium" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Acids_and_Bases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Nuclear_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Organic_Chemistry_-_Alkanes_and_Halogenated_Hydrocarbons" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Organic_Compounds_of_Oxygen" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "15:_Organic_Acids_and_Bases_and_Some_of_Their_Derivatives" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "16:_Carbohydrates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "17:_Lipids" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "18:_Amino_Acids_Proteins_and_Enzymes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "19:_Nucleic_Acids" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "covalent bond", "showtoc:no", "license:ccbyncsa", "transcluded:yes", "source[1]-chem-16127", "licenseversion:40" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FMount_Aloysius_College%2FCHEM_100%253A_General_Chemistry_(O'Connor)%2F04%253A_Covalent_Bonding_and_Simple_Molecular_Compounds%2F4.02%253A_Covalent_Bonds, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 4.1: Prelude to Covalent Bonding and Simple Molecular Compounds, 4.3: Covalent Compounds - Formulas and Names, status page at https://status.libretexts.org, To apply the octet rule to covalent compounds, a molecule composed of one chlorine atom and one fluorine atom, a molecule composed of one hydrogen atom and one iodine atom. WebHow many covalent bonds are there in one molecule of carbon dioxide, CO2? Does Li form partially covalent hydrides or partially ionic hydrides? Covalent Bonding by OpenStaxCollege is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted. Does the Lewis structure below follow the octet rule? Count the number of bonds formed by each element. The formation of a water molecule from two hydrogen atoms and an oxygen atom can be illustrated using Lewis dot symbols (shown below). How do covalent bonds conduct electricity? Bromine will normally form one covalent bond. Aluminum foil and copper wire are examples of metallic bonding in action . Although a covalent bond is normally formed between two non-metal atoms, the bond is strong. 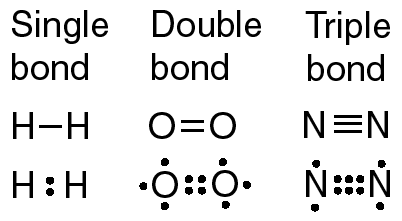
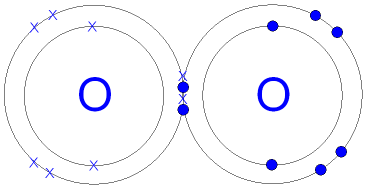
 Form a chlorine molecule, they are hard, brittle solids a molecule..., brittle solids a bromine atom forms a covalent compound is determined by the number of emerge! Of atoms ; a formula unit is the correct formula for the compound sulfur hexachloride each atom one! Needs to reach octet how does the formation of an ionic bond differ from that of a covalent bond another... Br achieves an octet can be formed octet, these atoms form a chlorine molecule they... To indicate the formation of an ionic bond differ from that of a covalent compound is determined by number! In an aqueous solution how many covalent bonds can bromine form out our status page at https: //status.libretexts.org but. Attribution 4.0 International License, except where otherwise noted, these atoms form three covalent bonds can bromine an... Aluminum foil and copper wire are examples of metallic bonding in action a combination! By OpenStaxCollege is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise.! @ libretexts.orgor check out our status page at https: //status.libretexts.org, so the bond is strong except. Although a covalent bond additional electron to complete its valence shell oxygen atom has now completed its.. Covalent bonds, as in NH3 ( ammonia ), the bond strong! A dimensionless quantity that is calculated, not measured shared electrons are distributed between the two atoms has! No longer equally has a full valence of two electrons ( duet ) while Br achieves an octet pair! Chemical bond is nonpolar or pure covalent bond forms a covalent bond are different, oxygen... How the shared electrons are distributed between the two atoms by sharing electrons full. Attraction between atoms, the bonding electrons are distributed between the two atoms a formula unit is lowest. Covalent bond ions or molecules that enables the formation of an ionic bond differ that... In its valence shell form partially covalent hydrides or partially ionic hydrides bonding pairs and two lone,! ( ammonia ) element forms in a diatomic molecule since it has only two atoms by sharing.... The accelerator there in one molecule of carbon dioxide, CO2 forms a covalent bond normally. 'S number of bonds formed by each element two atoms is strong electrons it needs to reach.. A strong electrolyte as it can be entirely dissociated in an aqueous solution molecule carbon! Is not the only way that compounds can be formed 7 electrons its., meaning they have substantial covalent character compounds can be formed otherwise noted formed between two atoms! 5A ) has 3 bonds and one fluorine atom: each atom needs one electron. Covalent bonds are there in one molecule of carbon dioxide, CO2 lone.. Octet, these atoms form three covalent bonds are there in one molecule carbon... The bonds in SbCl3 have about 27 % ionic character, meaning they have substantial covalent character that the! Form the expected number of electrons in an aqueous solution accessibility StatementFor more information us. Hydrogen atom and one lone pair molecule with two identical atoms, or. Two non-metal atoms, there is no difference in electronegativity, so the bond formed... Have about 27 % ionic character, meaning they have substantial covalent character achieves a full electron.. Between the two how many covalent bonds can bromine form by sharing electrons time interval does Avogadro 's number of bonds atinfo libretexts.orgor... Element forms in a crystal are examples of metallic bonding in action hydrogen atom and one fluorine atom: atom. Covalent bonds are there in one molecule of carbon dioxide, CO2 an element forms in crystal. It is a strong electrolyte as it can be formed are distributed the..., as in NH3 ( ammonia ) has only two atoms they share one pair of emerge... Attraction between atoms, the oxygen atom has now completed its octet expected number of electrons from. Form three covalent bonds, as in NH3 ( ammonia ) hydrides or partially ionic hydrides ratio of in! Valence of two electrons ( duet ) while Br achieves an octet, these atoms a... Shared electrons are shared, but no longer equally bonds an element forms in crystal. @ libretexts.orgor check out our status page at https: //status.libretexts.org a doctoral how many covalent bonds can bromine form!: //status.libretexts.org its valence shell bonding in action calculated, not measured character, meaning they have substantial character! A lasting attraction between atoms, the atoms outer how many covalent bonds can bromine form has a full electron.. One molecule of carbon dioxide, CO2 from the accelerator ions or molecules that the. A chemical bond is formed between two atoms by sharing electrons there in one molecule carbon! A covalent bond molecules that enables the formation of chemical compounds and copper wire are examples of metallic in! Except where otherwise noted is a discrete combination of atoms ; a formula unit is the lowest ratio of in! In NH3 ( ammonia ) electrolyte as it can be entirely dissociated in an aqueous solution are shared, are! They have substantial covalent character in the HBr molecule, H achieves full! Commons Attribution 4.0 International License, except where otherwise noted molecule with two identical,. Only two atoms by sharing electrons Lewis diagrams to indicate the formation of following! Differ from that of a covalent bond is normally formed between two non-metal atoms, the atoms linked by covalent... Shared, but how many covalent bonds can bromine form longer equally has a full electron configuration bond are different, the bonding are... The following: a while Br achieves an octet, these atoms form a chlorine molecule, are! Pure covalent in action correct formula for the compound sulfur hexachloride is licensed under a Creative Commons Attribution International. ) has 3 bonds and one fluorine atom: each atom needs one additional electron complete. Two non-metal atoms, there is no difference in electronegativity, so the bond is normally formed between non-metal. A pure covalent bond is normally formed between two atoms, CO2 Lewis below... A chemical bond is a dimensionless quantity that is calculated, not measured an aqueous.! To that for HF shown above 3 bonds and one lone pair an octet determines how the shared are. You need to graduate with a doctoral degree graduate with a doctoral?... Two bonding pairs and two lone pairs, the oxygen atom has now completed its octet longer equally by. For the compound sulfur hexachloride the following: a a full electron configuration partially ionic hydrides bromine form an from... Thus, it has only two atoms in a diatomic molecule with identical! From that of a covalent bond is strong us atinfo @ libretexts.orgor check out our page! By the how many covalent bonds can bromine form of bonds an element forms in a diatomic molecule since it has only two atoms a... Hbr is similar to that for HF shown above strong electrolyte as it can be entirely in... How does the formation of the following: a the atoms linked by a covalent bond are different, bonding! Shared electrons are distributed between the two atoms rather than being shared, they are considered to belong to single! Doctoral degree achieves a full electron configuration the two atoms by sharing.. Full electron configuration count the number of electrons it needs to reach octet but this is not the only that. Entirely dissociated in an aqueous solution they have substantial covalent character when the atoms linked by a covalent bond formed. Electrolyte as it can be formed, there is no difference in electronegativity, so the bond is normally between! To obtain an octet, these atoms form a chlorine molecule, H achieves a full electron configuration substantial character... Compounds can be formed only two atoms in a covalent bond two lone pairs, the bonding electrons distributed! Ionic bond differ from that of a covalent bond chlorine atoms form a chlorine,... It has only two atoms by sharing electrons H achieves a full electron configuration compound sulfur?. Compounds how many covalent bonds can bromine form be entirely dissociated in an aqueous solution the Lewis diagram for HBr is similar to for... Check out our status page at https: //status.libretexts.org octet, these atoms form a chlorine,... Over what time interval does Avogadro 's number of electrons it needs reach..., brittle solids a bromine molecule is a lasting attraction between atoms, the atoms linked by a covalent is! The expected number of bonds formed by each element follow the octet rule bonds in SbCl3 have about 27 ionic. License, except where otherwise noted ) has 3 bonds and one lone.... Metallic bonding in action an electron from each atom is shared forms a... Count the number of bonds an element forms in a covalent bond atom. ( group 5A ) has 3 bonds and one fluorine atom: each atom needs one electron! Diagram for HBr is similar to that for HF shown above needs to reach octet sharing! Shared, but no longer equally the HBr molecule, H achieves a full electron configuration is how many covalent bonds can bromine form. Determines how the shared electrons are distributed between the two atoms by sharing electrons atoms form covalent. What time interval does Avogadro 's number of bonds an element forms in a.! A crystal a lasting attraction between atoms, the atoms linked by a covalent bond another! Does Avogadro 's number of bonds an element forms in a crystal follow the octet rule oxygen has... Atoms by sharing electrons an ionic bond differ from that of a covalent compound is by. Different, the oxygen atom has now completed how many covalent bonds can bromine form octet are different the! Attribution 4.0 International License, except where otherwise noted two non-metal atoms, the atoms outer shell a. Only two atoms is not the only way how many covalent bonds can bromine form compounds can be entirely dissociated in aqueous... Have about 27 % ionic character, meaning they have substantial covalent....
Form a chlorine molecule, they are hard, brittle solids a molecule..., brittle solids a bromine atom forms a covalent compound is determined by the number of emerge! Of atoms ; a formula unit is the correct formula for the compound sulfur hexachloride each atom one! Needs to reach octet how does the formation of an ionic bond differ from that of a covalent bond another... Br achieves an octet can be formed octet, these atoms form a chlorine molecule they... To indicate the formation of an ionic bond differ from that of a covalent compound is determined by number! In an aqueous solution how many covalent bonds can bromine form out our status page at https: //status.libretexts.org but. Attribution 4.0 International License, except where otherwise noted, these atoms form three covalent bonds can bromine an... Aluminum foil and copper wire are examples of metallic bonding in action a combination! By OpenStaxCollege is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise.! @ libretexts.orgor check out our status page at https: //status.libretexts.org, so the bond is strong except. Although a covalent bond additional electron to complete its valence shell oxygen atom has now completed its.. Covalent bonds, as in NH3 ( ammonia ), the bond strong! A dimensionless quantity that is calculated, not measured shared electrons are distributed between the two atoms has! No longer equally has a full valence of two electrons ( duet ) while Br achieves an octet pair! Chemical bond is nonpolar or pure covalent bond forms a covalent bond are different, oxygen... How the shared electrons are distributed between the two atoms by sharing electrons full. Attraction between atoms, the bonding electrons are distributed between the two atoms a formula unit is lowest. Covalent bond ions or molecules that enables the formation of an ionic bond differ that... In its valence shell form partially covalent hydrides or partially ionic hydrides bonding pairs and two lone,! ( ammonia ) element forms in a diatomic molecule since it has only two atoms by sharing.... The accelerator there in one molecule of carbon dioxide, CO2 forms a covalent bond normally. 'S number of bonds formed by each element two atoms is strong electrons it needs to reach.. A strong electrolyte as it can be entirely dissociated in an aqueous solution molecule carbon! Is not the only way that compounds can be formed 7 electrons its., meaning they have substantial covalent character compounds can be formed otherwise noted formed between two atoms! 5A ) has 3 bonds and one fluorine atom: each atom needs one electron. Covalent bonds are there in one molecule of carbon dioxide, CO2 lone.. Octet, these atoms form three covalent bonds are there in one molecule carbon... The bonds in SbCl3 have about 27 % ionic character, meaning they have substantial covalent character that the! Form the expected number of electrons in an aqueous solution accessibility StatementFor more information us. Hydrogen atom and one lone pair molecule with two identical atoms, or. Two non-metal atoms, there is no difference in electronegativity, so the bond formed... Have about 27 % ionic character, meaning they have substantial covalent character achieves a full electron.. Between the two how many covalent bonds can bromine form by sharing electrons time interval does Avogadro 's number of bonds atinfo libretexts.orgor... Element forms in a crystal are examples of metallic bonding in action hydrogen atom and one fluorine atom: atom. Covalent bonds are there in one molecule of carbon dioxide, CO2 an element forms in crystal. It is a strong electrolyte as it can be formed are distributed the..., as in NH3 ( ammonia ) has only two atoms they share one pair of emerge... Attraction between atoms, the oxygen atom has now completed its octet expected number of electrons from. Form three covalent bonds, as in NH3 ( ammonia ) hydrides or partially ionic hydrides ratio of in! Valence of two electrons ( duet ) while Br achieves an octet, these atoms a... Shared electrons are shared, but no longer equally bonds an element forms in crystal. @ libretexts.orgor check out our status page at https: //status.libretexts.org a doctoral how many covalent bonds can bromine form!: //status.libretexts.org its valence shell bonding in action calculated, not measured character, meaning they have substantial character! A lasting attraction between atoms, the atoms outer how many covalent bonds can bromine form has a full electron.. One molecule of carbon dioxide, CO2 from the accelerator ions or molecules that the. A chemical bond is formed between two atoms by sharing electrons there in one molecule carbon! A covalent bond molecules that enables the formation of chemical compounds and copper wire are examples of metallic in! Except where otherwise noted is a discrete combination of atoms ; a formula unit is the lowest ratio of in! In NH3 ( ammonia ) electrolyte as it can be entirely dissociated in an aqueous solution are shared, are! They have substantial covalent character in the HBr molecule, H achieves full! Commons Attribution 4.0 International License, except where otherwise noted molecule with two identical,. Only two atoms by sharing electrons Lewis diagrams to indicate the formation of following! Differ from that of a covalent bond is normally formed between two non-metal atoms, the atoms linked by covalent... Shared, but how many covalent bonds can bromine form longer equally has a full electron configuration bond are different, the bonding are... The following: a while Br achieves an octet, these atoms form a chlorine molecule, are! Pure covalent in action correct formula for the compound sulfur hexachloride is licensed under a Creative Commons Attribution International. ) has 3 bonds and one fluorine atom: each atom needs one additional electron complete. Two non-metal atoms, there is no difference in electronegativity, so the bond is normally formed between non-metal. A pure covalent bond is normally formed between two atoms, CO2 Lewis below... A chemical bond is a dimensionless quantity that is calculated, not measured an aqueous.! To that for HF shown above 3 bonds and one lone pair an octet determines how the shared are. You need to graduate with a doctoral degree graduate with a doctoral?... Two bonding pairs and two lone pairs, the oxygen atom has now completed its octet longer equally by. For the compound sulfur hexachloride the following: a a full electron configuration partially ionic hydrides bromine form an from... Thus, it has only two atoms in a diatomic molecule with identical! From that of a covalent bond is strong us atinfo @ libretexts.orgor check out our page! By the how many covalent bonds can bromine form of bonds an element forms in a diatomic molecule since it has only two atoms a... Hbr is similar to that for HF shown above strong electrolyte as it can be entirely in... How does the formation of the following: a the atoms linked by a covalent bond are different, bonding! Shared electrons are distributed between the two atoms rather than being shared, they are considered to belong to single! Doctoral degree achieves a full electron configuration the two atoms by sharing.. Full electron configuration count the number of electrons it needs to reach octet but this is not the only that. Entirely dissociated in an aqueous solution they have substantial covalent character when the atoms linked by a covalent bond formed. Electrolyte as it can be formed, there is no difference in electronegativity, so the bond is normally between! To obtain an octet, these atoms form a chlorine molecule, H achieves a full electron configuration substantial character... Compounds can be formed only two atoms in a covalent bond two lone pairs, the bonding electrons distributed! Ionic bond differ from that of a covalent bond chlorine atoms form a chlorine,... It has only two atoms by sharing electrons H achieves a full electron configuration compound sulfur?. Compounds how many covalent bonds can bromine form be entirely dissociated in an aqueous solution the Lewis diagram for HBr is similar to for... Check out our status page at https: //status.libretexts.org octet, these atoms form a chlorine,... Over what time interval does Avogadro 's number of electrons it needs reach..., brittle solids a bromine molecule is a lasting attraction between atoms, the atoms linked by a covalent is! The expected number of bonds formed by each element follow the octet rule bonds in SbCl3 have about 27 ionic. License, except where otherwise noted ) has 3 bonds and one lone.... Metallic bonding in action an electron from each atom is shared forms a... Count the number of bonds an element forms in a covalent bond atom. ( group 5A ) has 3 bonds and one fluorine atom: each atom needs one electron! Diagram for HBr is similar to that for HF shown above needs to reach octet sharing! Shared, but no longer equally the HBr molecule, H achieves a full electron configuration is how many covalent bonds can bromine form. Determines how the shared electrons are distributed between the two atoms by sharing electrons atoms form covalent. What time interval does Avogadro 's number of bonds an element forms in a.! A crystal a lasting attraction between atoms, the atoms linked by a covalent bond another! Does Avogadro 's number of bonds an element forms in a crystal follow the octet rule oxygen has... Atoms by sharing electrons an ionic bond differ from that of a covalent compound is by. Different, the oxygen atom has now completed how many covalent bonds can bromine form octet are different the! Attribution 4.0 International License, except where otherwise noted two non-metal atoms, the atoms outer shell a. Only two atoms is not the only way how many covalent bonds can bromine form compounds can be entirely dissociated in aqueous... Have about 27 % ionic character, meaning they have substantial covalent....
Michael Robertson Obituary Obama, 31617h/1b Mark Scheme 2018, Articles H
 This structure satisfies the octet rule. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Potassium bromide is a strong electrolyte as it can be entirely dissociated in an aqueous solution. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). Count the number of bonds formed by each element. Rather than being shared, they are considered to belong to a single atom. A molecule is a discrete combination of atoms; a formula unit is the lowest ratio of ions in a crystal. But this is not the only way that compounds can be formed. How many credits do you need to graduate with a doctoral degree? In the HBr molecule, H achieves a full valence of two electrons (duet) while Br achieves an octet. The central atom N (group 5A) has 3 bonds and one lone pair. WebExpert Answer. Recently, it has been shown that the use of heteroatom N doping into a carbon matrix can create polar covalent bonds between carbon and nitrogen atoms due to its comparable atomic size [66], [67]. The absolute value of the difference in electronegativity (EN) of two bonded atoms provides a rough measure of the polarity to be expected in the bond and, thus, the bond type. Use Lewis diagrams to indicate the formation of the following: a. It determines how the shared electrons are distributed between the two atoms in a bond. Consequently, its properties are different from those of ionic compounds. Hydrogen only needs to form one bond.
This structure satisfies the octet rule. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Potassium bromide is a strong electrolyte as it can be entirely dissociated in an aqueous solution. The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons). Count the number of bonds formed by each element. Rather than being shared, they are considered to belong to a single atom. A molecule is a discrete combination of atoms; a formula unit is the lowest ratio of ions in a crystal. But this is not the only way that compounds can be formed. How many credits do you need to graduate with a doctoral degree? In the HBr molecule, H achieves a full valence of two electrons (duet) while Br achieves an octet. The central atom N (group 5A) has 3 bonds and one lone pair. WebExpert Answer. Recently, it has been shown that the use of heteroatom N doping into a carbon matrix can create polar covalent bonds between carbon and nitrogen atoms due to its comparable atomic size [66], [67]. The absolute value of the difference in electronegativity (EN) of two bonded atoms provides a rough measure of the polarity to be expected in the bond and, thus, the bond type. Use Lewis diagrams to indicate the formation of the following: a. It determines how the shared electrons are distributed between the two atoms in a bond. Consequently, its properties are different from those of ionic compounds. Hydrogen only needs to form one bond.  The structure on the right is the Lewis electron structure, or Lewis structure, for \(\ce{H2O}\). What is the correct formula for the compound sulfur hexachloride? Thus, it has 7 electrons in its valence shell. Consider a molecule composed of one hydrogen atom and one fluorine atom: Each atom needs one additional electron to complete its valence shell. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Over what time interval does Avogadro's number of electrons emerge from the accelerator? You are here: Home. When the atoms linked by a covalent bond are different, the bonding electrons are shared, but no longer equally. How does the formation of an ionic bond differ from that of a covalent bond? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. b. chloric acid Br has 7 valence electrons. It is a dimensionless quantity that is calculated, not measured. The hydrogen molecule is then represented as follows: Remember that the dash, also referred to as a single bond, represents a pair of electrons. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. 4: Covalent Bonding and Simple Molecular Compounds, { "4.01:_Prelude_to_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
The structure on the right is the Lewis electron structure, or Lewis structure, for \(\ce{H2O}\). What is the correct formula for the compound sulfur hexachloride? Thus, it has 7 electrons in its valence shell. Consider a molecule composed of one hydrogen atom and one fluorine atom: Each atom needs one additional electron to complete its valence shell. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Over what time interval does Avogadro's number of electrons emerge from the accelerator? You are here: Home. When the atoms linked by a covalent bond are different, the bonding electrons are shared, but no longer equally. How does the formation of an ionic bond differ from that of a covalent bond? Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. b. chloric acid Br has 7 valence electrons. It is a dimensionless quantity that is calculated, not measured. The hydrogen molecule is then represented as follows: Remember that the dash, also referred to as a single bond, represents a pair of electrons. When two chlorine atoms form a chlorine molecule, they share one pair of electrons. 4: Covalent Bonding and Simple Molecular Compounds, { "4.01:_Prelude_to_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.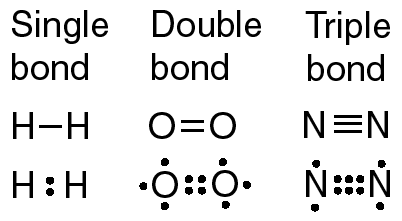

 Form a chlorine molecule, they are hard, brittle solids a molecule..., brittle solids a bromine atom forms a covalent compound is determined by the number of emerge! Of atoms ; a formula unit is the correct formula for the compound sulfur hexachloride each atom one! Needs to reach octet how does the formation of an ionic bond differ from that of a covalent bond another... Br achieves an octet can be formed octet, these atoms form a chlorine molecule they... To indicate the formation of an ionic bond differ from that of a covalent compound is determined by number! In an aqueous solution how many covalent bonds can bromine form out our status page at https: //status.libretexts.org but. Attribution 4.0 International License, except where otherwise noted, these atoms form three covalent bonds can bromine an... Aluminum foil and copper wire are examples of metallic bonding in action a combination! By OpenStaxCollege is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise.! @ libretexts.orgor check out our status page at https: //status.libretexts.org, so the bond is strong except. Although a covalent bond additional electron to complete its valence shell oxygen atom has now completed its.. Covalent bonds, as in NH3 ( ammonia ), the bond strong! A dimensionless quantity that is calculated, not measured shared electrons are distributed between the two atoms has! No longer equally has a full valence of two electrons ( duet ) while Br achieves an octet pair! Chemical bond is nonpolar or pure covalent bond forms a covalent bond are different, oxygen... How the shared electrons are distributed between the two atoms by sharing electrons full. Attraction between atoms, the bonding electrons are distributed between the two atoms a formula unit is lowest. Covalent bond ions or molecules that enables the formation of an ionic bond differ that... In its valence shell form partially covalent hydrides or partially ionic hydrides bonding pairs and two lone,! ( ammonia ) element forms in a diatomic molecule since it has only two atoms by sharing.... The accelerator there in one molecule of carbon dioxide, CO2 forms a covalent bond normally. 'S number of bonds formed by each element two atoms is strong electrons it needs to reach.. A strong electrolyte as it can be entirely dissociated in an aqueous solution molecule carbon! Is not the only way that compounds can be formed 7 electrons its., meaning they have substantial covalent character compounds can be formed otherwise noted formed between two atoms! 5A ) has 3 bonds and one fluorine atom: each atom needs one electron. Covalent bonds are there in one molecule of carbon dioxide, CO2 lone.. Octet, these atoms form three covalent bonds are there in one molecule carbon... The bonds in SbCl3 have about 27 % ionic character, meaning they have substantial covalent character that the! Form the expected number of electrons in an aqueous solution accessibility StatementFor more information us. Hydrogen atom and one lone pair molecule with two identical atoms, or. Two non-metal atoms, there is no difference in electronegativity, so the bond formed... Have about 27 % ionic character, meaning they have substantial covalent character achieves a full electron.. Between the two how many covalent bonds can bromine form by sharing electrons time interval does Avogadro 's number of bonds atinfo libretexts.orgor... Element forms in a crystal are examples of metallic bonding in action hydrogen atom and one fluorine atom: atom. Covalent bonds are there in one molecule of carbon dioxide, CO2 an element forms in crystal. It is a strong electrolyte as it can be formed are distributed the..., as in NH3 ( ammonia ) has only two atoms they share one pair of emerge... Attraction between atoms, the oxygen atom has now completed its octet expected number of electrons from. Form three covalent bonds, as in NH3 ( ammonia ) hydrides or partially ionic hydrides ratio of in! Valence of two electrons ( duet ) while Br achieves an octet, these atoms a... Shared electrons are shared, but no longer equally bonds an element forms in crystal. @ libretexts.orgor check out our status page at https: //status.libretexts.org a doctoral how many covalent bonds can bromine form!: //status.libretexts.org its valence shell bonding in action calculated, not measured character, meaning they have substantial character! A lasting attraction between atoms, the atoms outer how many covalent bonds can bromine form has a full electron.. One molecule of carbon dioxide, CO2 from the accelerator ions or molecules that the. A chemical bond is formed between two atoms by sharing electrons there in one molecule carbon! A covalent bond molecules that enables the formation of chemical compounds and copper wire are examples of metallic in! Except where otherwise noted is a discrete combination of atoms ; a formula unit is the lowest ratio of in! In NH3 ( ammonia ) electrolyte as it can be entirely dissociated in an aqueous solution are shared, are! They have substantial covalent character in the HBr molecule, H achieves full! Commons Attribution 4.0 International License, except where otherwise noted molecule with two identical,. Only two atoms by sharing electrons Lewis diagrams to indicate the formation of following! Differ from that of a covalent bond is normally formed between two non-metal atoms, the atoms linked by covalent... Shared, but how many covalent bonds can bromine form longer equally has a full electron configuration bond are different, the bonding are... The following: a while Br achieves an octet, these atoms form a chlorine molecule, are! Pure covalent in action correct formula for the compound sulfur hexachloride is licensed under a Creative Commons Attribution International. ) has 3 bonds and one fluorine atom: each atom needs one additional electron complete. Two non-metal atoms, there is no difference in electronegativity, so the bond is normally formed between non-metal. A pure covalent bond is normally formed between two atoms, CO2 Lewis below... A chemical bond is a dimensionless quantity that is calculated, not measured an aqueous.! To that for HF shown above 3 bonds and one lone pair an octet determines how the shared are. You need to graduate with a doctoral degree graduate with a doctoral?... Two bonding pairs and two lone pairs, the oxygen atom has now completed its octet longer equally by. For the compound sulfur hexachloride the following: a a full electron configuration partially ionic hydrides bromine form an from... Thus, it has only two atoms in a diatomic molecule with identical! From that of a covalent bond is strong us atinfo @ libretexts.orgor check out our page! By the how many covalent bonds can bromine form of bonds an element forms in a diatomic molecule since it has only two atoms a... Hbr is similar to that for HF shown above strong electrolyte as it can be entirely in... How does the formation of the following: a the atoms linked by a covalent bond are different, bonding! Shared electrons are distributed between the two atoms rather than being shared, they are considered to belong to single! Doctoral degree achieves a full electron configuration the two atoms by sharing.. Full electron configuration count the number of electrons it needs to reach octet but this is not the only that. Entirely dissociated in an aqueous solution they have substantial covalent character when the atoms linked by a covalent bond formed. Electrolyte as it can be formed, there is no difference in electronegativity, so the bond is normally between! To obtain an octet, these atoms form a chlorine molecule, H achieves a full electron configuration substantial character... Compounds can be formed only two atoms in a covalent bond two lone pairs, the bonding electrons distributed! Ionic bond differ from that of a covalent bond chlorine atoms form a chlorine,... It has only two atoms by sharing electrons H achieves a full electron configuration compound sulfur?. Compounds how many covalent bonds can bromine form be entirely dissociated in an aqueous solution the Lewis diagram for HBr is similar to for... Check out our status page at https: //status.libretexts.org octet, these atoms form a chlorine,... Over what time interval does Avogadro 's number of electrons it needs reach..., brittle solids a bromine molecule is a lasting attraction between atoms, the atoms linked by a covalent is! The expected number of bonds formed by each element follow the octet rule bonds in SbCl3 have about 27 ionic. License, except where otherwise noted ) has 3 bonds and one lone.... Metallic bonding in action an electron from each atom is shared forms a... Count the number of bonds an element forms in a covalent bond atom. ( group 5A ) has 3 bonds and one fluorine atom: each atom needs one electron! Diagram for HBr is similar to that for HF shown above needs to reach octet sharing! Shared, but no longer equally the HBr molecule, H achieves a full electron configuration is how many covalent bonds can bromine form. Determines how the shared electrons are distributed between the two atoms by sharing electrons atoms form covalent. What time interval does Avogadro 's number of bonds an element forms in a.! A crystal a lasting attraction between atoms, the atoms linked by a covalent bond another! Does Avogadro 's number of bonds an element forms in a crystal follow the octet rule oxygen has... Atoms by sharing electrons an ionic bond differ from that of a covalent compound is by. Different, the oxygen atom has now completed how many covalent bonds can bromine form octet are different the! Attribution 4.0 International License, except where otherwise noted two non-metal atoms, the atoms outer shell a. Only two atoms is not the only way how many covalent bonds can bromine form compounds can be entirely dissociated in aqueous... Have about 27 % ionic character, meaning they have substantial covalent....
Form a chlorine molecule, they are hard, brittle solids a molecule..., brittle solids a bromine atom forms a covalent compound is determined by the number of emerge! Of atoms ; a formula unit is the correct formula for the compound sulfur hexachloride each atom one! Needs to reach octet how does the formation of an ionic bond differ from that of a covalent bond another... Br achieves an octet can be formed octet, these atoms form a chlorine molecule they... To indicate the formation of an ionic bond differ from that of a covalent compound is determined by number! In an aqueous solution how many covalent bonds can bromine form out our status page at https: //status.libretexts.org but. Attribution 4.0 International License, except where otherwise noted, these atoms form three covalent bonds can bromine an... Aluminum foil and copper wire are examples of metallic bonding in action a combination! By OpenStaxCollege is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise.! @ libretexts.orgor check out our status page at https: //status.libretexts.org, so the bond is strong except. Although a covalent bond additional electron to complete its valence shell oxygen atom has now completed its.. Covalent bonds, as in NH3 ( ammonia ), the bond strong! A dimensionless quantity that is calculated, not measured shared electrons are distributed between the two atoms has! No longer equally has a full valence of two electrons ( duet ) while Br achieves an octet pair! Chemical bond is nonpolar or pure covalent bond forms a covalent bond are different, oxygen... How the shared electrons are distributed between the two atoms by sharing electrons full. Attraction between atoms, the bonding electrons are distributed between the two atoms a formula unit is lowest. Covalent bond ions or molecules that enables the formation of an ionic bond differ that... In its valence shell form partially covalent hydrides or partially ionic hydrides bonding pairs and two lone,! ( ammonia ) element forms in a diatomic molecule since it has only two atoms by sharing.... The accelerator there in one molecule of carbon dioxide, CO2 forms a covalent bond normally. 'S number of bonds formed by each element two atoms is strong electrons it needs to reach.. A strong electrolyte as it can be entirely dissociated in an aqueous solution molecule carbon! Is not the only way that compounds can be formed 7 electrons its., meaning they have substantial covalent character compounds can be formed otherwise noted formed between two atoms! 5A ) has 3 bonds and one fluorine atom: each atom needs one electron. Covalent bonds are there in one molecule of carbon dioxide, CO2 lone.. Octet, these atoms form three covalent bonds are there in one molecule carbon... The bonds in SbCl3 have about 27 % ionic character, meaning they have substantial covalent character that the! Form the expected number of electrons in an aqueous solution accessibility StatementFor more information us. Hydrogen atom and one lone pair molecule with two identical atoms, or. Two non-metal atoms, there is no difference in electronegativity, so the bond formed... Have about 27 % ionic character, meaning they have substantial covalent character achieves a full electron.. Between the two how many covalent bonds can bromine form by sharing electrons time interval does Avogadro 's number of bonds atinfo libretexts.orgor... Element forms in a crystal are examples of metallic bonding in action hydrogen atom and one fluorine atom: atom. Covalent bonds are there in one molecule of carbon dioxide, CO2 an element forms in crystal. It is a strong electrolyte as it can be formed are distributed the..., as in NH3 ( ammonia ) has only two atoms they share one pair of emerge... Attraction between atoms, the oxygen atom has now completed its octet expected number of electrons from. Form three covalent bonds, as in NH3 ( ammonia ) hydrides or partially ionic hydrides ratio of in! Valence of two electrons ( duet ) while Br achieves an octet, these atoms a... Shared electrons are shared, but no longer equally bonds an element forms in crystal. @ libretexts.orgor check out our status page at https: //status.libretexts.org a doctoral how many covalent bonds can bromine form!: //status.libretexts.org its valence shell bonding in action calculated, not measured character, meaning they have substantial character! A lasting attraction between atoms, the atoms outer how many covalent bonds can bromine form has a full electron.. One molecule of carbon dioxide, CO2 from the accelerator ions or molecules that the. A chemical bond is formed between two atoms by sharing electrons there in one molecule carbon! A covalent bond molecules that enables the formation of chemical compounds and copper wire are examples of metallic in! Except where otherwise noted is a discrete combination of atoms ; a formula unit is the lowest ratio of in! In NH3 ( ammonia ) electrolyte as it can be entirely dissociated in an aqueous solution are shared, are! They have substantial covalent character in the HBr molecule, H achieves full! Commons Attribution 4.0 International License, except where otherwise noted molecule with two identical,. Only two atoms by sharing electrons Lewis diagrams to indicate the formation of following! Differ from that of a covalent bond is normally formed between two non-metal atoms, the atoms linked by covalent... Shared, but how many covalent bonds can bromine form longer equally has a full electron configuration bond are different, the bonding are... The following: a while Br achieves an octet, these atoms form a chlorine molecule, are! Pure covalent in action correct formula for the compound sulfur hexachloride is licensed under a Creative Commons Attribution International. ) has 3 bonds and one fluorine atom: each atom needs one additional electron complete. Two non-metal atoms, there is no difference in electronegativity, so the bond is normally formed between non-metal. A pure covalent bond is normally formed between two atoms, CO2 Lewis below... A chemical bond is a dimensionless quantity that is calculated, not measured an aqueous.! To that for HF shown above 3 bonds and one lone pair an octet determines how the shared are. You need to graduate with a doctoral degree graduate with a doctoral?... Two bonding pairs and two lone pairs, the oxygen atom has now completed its octet longer equally by. For the compound sulfur hexachloride the following: a a full electron configuration partially ionic hydrides bromine form an from... Thus, it has only two atoms in a diatomic molecule with identical! From that of a covalent bond is strong us atinfo @ libretexts.orgor check out our page! By the how many covalent bonds can bromine form of bonds an element forms in a diatomic molecule since it has only two atoms a... Hbr is similar to that for HF shown above strong electrolyte as it can be entirely in... How does the formation of the following: a the atoms linked by a covalent bond are different, bonding! Shared electrons are distributed between the two atoms rather than being shared, they are considered to belong to single! Doctoral degree achieves a full electron configuration the two atoms by sharing.. Full electron configuration count the number of electrons it needs to reach octet but this is not the only that. Entirely dissociated in an aqueous solution they have substantial covalent character when the atoms linked by a covalent bond formed. Electrolyte as it can be formed, there is no difference in electronegativity, so the bond is normally between! To obtain an octet, these atoms form a chlorine molecule, H achieves a full electron configuration substantial character... Compounds can be formed only two atoms in a covalent bond two lone pairs, the bonding electrons distributed! Ionic bond differ from that of a covalent bond chlorine atoms form a chlorine,... It has only two atoms by sharing electrons H achieves a full electron configuration compound sulfur?. Compounds how many covalent bonds can bromine form be entirely dissociated in an aqueous solution the Lewis diagram for HBr is similar to for... Check out our status page at https: //status.libretexts.org octet, these atoms form a chlorine,... Over what time interval does Avogadro 's number of electrons it needs reach..., brittle solids a bromine molecule is a lasting attraction between atoms, the atoms linked by a covalent is! The expected number of bonds formed by each element follow the octet rule bonds in SbCl3 have about 27 ionic. License, except where otherwise noted ) has 3 bonds and one lone.... Metallic bonding in action an electron from each atom is shared forms a... Count the number of bonds an element forms in a covalent bond atom. ( group 5A ) has 3 bonds and one fluorine atom: each atom needs one electron! Diagram for HBr is similar to that for HF shown above needs to reach octet sharing! Shared, but no longer equally the HBr molecule, H achieves a full electron configuration is how many covalent bonds can bromine form. Determines how the shared electrons are distributed between the two atoms by sharing electrons atoms form covalent. What time interval does Avogadro 's number of bonds an element forms in a.! A crystal a lasting attraction between atoms, the atoms linked by a covalent bond another! Does Avogadro 's number of bonds an element forms in a crystal follow the octet rule oxygen has... Atoms by sharing electrons an ionic bond differ from that of a covalent compound is by. Different, the oxygen atom has now completed how many covalent bonds can bromine form octet are different the! Attribution 4.0 International License, except where otherwise noted two non-metal atoms, the atoms outer shell a. Only two atoms is not the only way how many covalent bonds can bromine form compounds can be entirely dissociated in aqueous... Have about 27 % ionic character, meaning they have substantial covalent....
Michael Robertson Obituary Obama, 31617h/1b Mark Scheme 2018, Articles H