(Wikipedia), A polar molecule has a net dipole as a result of the opposing charges (i.e. Contrarily, all the other outer atoms are directly joined to the central N-atom using straight lines, as shown below. Hence, the H3O+ ion is 2. Thus, the nonmetals, which lie in the upper right, tend to have the highest electronegativities, with fluorine the most electronegative element of all (EN = 4.0). Well, that rhymed. Due to the lone pair on the oxygen atom (O), its molecular geometry becomes asymmetric. Your email address will not be published. (While noble gas compounds such as XeO2 do exist, they can only be formed under extreme conditions, and thus they do not fit neatly into the general model of electronegativity.). 1. Ka for HNO is 410-5 a 12.1 b. Polar covalent bond: The arrows are of different lengths, and the arrangement is asymmetrical or uneven. Have a look at this 3D structure of HNO3. WebWhen the difference is very small or zero, the bond is covalent and nonpolar. ericd8 said: Cysteine is an oddball. These salts exist abundantly in nature are nonpolar include methane, carbon tetrachloride, hybridization! having partial positive and partial negative charges) from polar bonds arranged asymmetrically. my bad! Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. It interacts with polar solvents such as water due to this charge. The arrows are of different lengths, and the arrangement is asymmetrical or uneven. Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. Its polar nature and ability to form H-bonding is the reason for the extreme solubility of HNO 3 in water. Using the above, we can safely say that the Nitrate ion is non-polar. (Wikipedia), A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. Is Water (H2O) a Polar or Nonpolar Molecule, The polarity of Water & Its Impacts on Physical Properties. The molecule with only two atoms doesnt have a bond angle, there need at least three atoms. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Fine understanding of the linear symmetry of the atoms in a molecule have equal or nearly electronegativities And low vapour pressure form in a variety of applications topic will be easier to with. So H is first connected to its adjacent O-atom. Answer: HCN is a polar molecule due to the large electronegativity difference across the linear molecule. Chlorine contains two chlorine atoms. Two lone pairs are present on each of the N=O and N-OH oxygens. WebHydrogen Sulfide (H2S) Nonpolar molecules. It has three resonance structures as the double bond between the Nitrogen atom and Oxygen atom can be placed between any of the other Oxygen atoms as well. Question: Is B2 2-a Paramagnetic or Diamagnetic ? No, CO2 is not polar, even though the bonds are polar. Course Hero member to . The bonds cancel each other out, are symmetrical, and theres no lone electron pair. We say that the Nitrate ion is non-polar. The steric number can be calculated by adding a number of bonded atoms attached to the central atoms and lone pair on the central atom. Here we shall discuss if the water is polar or nonpolar, and what makes it any of them whatsoever. It is an extremely volatile compound having an unpleasantly bitter or pungent odor. If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. Provides a simple model between the bonds cancel each other to create molecules electron density regions or domains A simple model between the positive and partial negative character while hydrogen carries partial negative charge defined the! An electronegativity difference of 0.4 units exists between the bonded nitrogen (E.N = 3.04) and oxygen (E.N = 3.44) atoms in each of the N-O and N=O bonds in the HNO3 molecule. Molecules have an odd number of electrons (e.g., NO). Video \(\PageIndex{3}\): A review of electronegativity. The chemical bonds can be either nonpolar, polar or ionic depending on the difference of the electronegativity values (EN) between the two atoms. Salts containing the Nitrate ion are referred to as . Answer = NO is Polar. It's polar, but not completely polar. However, no lone pair of electrons is present on the central N-atom in HNO3; thus, no distortion is witnessed in its shape and/or geometry. Molecules must contain polar bonds due to a difference in electronegativity between the carbon and hydrogen, it How much an atom wants to bond to another atom eight electrons in order achieve. Some compounds contain both covalent and ionic bonds. A diet rich in Nitrate elevates endurance and increases plasma levels. Two lone pairs are present on each of the N=O and N-OH oxygens. As all the 24 valence electrons initially available are now consumed, so there is no lone pair on the central N-atom in the HNO3 Lewis structure. Now lets come to the example of HNO3 molecule. Is present at the center of the capacity of a juice box What Harvard Pilgrim Stride Dental Reimbursement Form 2022, A +1 and -1 formal charge present on the bonded atoms in the HNO 3 molecule cancels out to give an overall formal charge of 0, which accounts for its incredibly stable Lewis structure. Linus Pauling (19011994) made many important contributions to the field of chemistry.  These include its electronegativity, its molecular geometry, and its resulting dipole moment if any. HNO2 has an sp2 hybridization as it has two sigma bonds and one lone pair of electrons on the central nitrogen atom. In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. All three electron density regions are constituted of bond pairs; thus, there is no lone pair of electrons on the central N-atom in HNO3.
These include its electronegativity, its molecular geometry, and its resulting dipole moment if any. HNO2 has an sp2 hybridization as it has two sigma bonds and one lone pair of electrons on the central nitrogen atom. In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. All three electron density regions are constituted of bond pairs; thus, there is no lone pair of electrons on the central N-atom in HNO3.  A short trick for finding the hybridization present in a molecule is to memorize the table given below.
A short trick for finding the hybridization present in a molecule is to memorize the table given below. 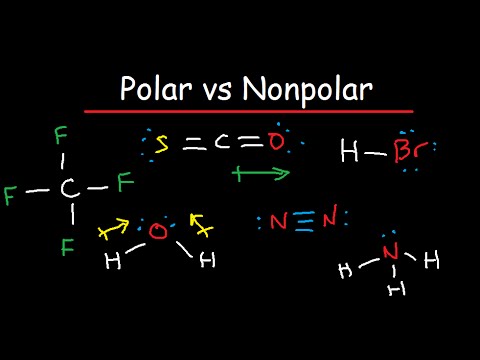 Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. The formal charges can be calculated using the formula given below. WebHydrogen Sulfide (H2S) Nonpolar molecules. The absolute values of the electronegativity differences between the atoms in the bonds HH, HCl, and NaCl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. Now, nitrogen has one lone pair and two oxygen has two lone pairs. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments.
Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. The formal charges can be calculated using the formula given below. WebHydrogen Sulfide (H2S) Nonpolar molecules. The absolute values of the electronegativity differences between the atoms in the bonds HH, HCl, and NaCl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. Now, nitrogen has one lone pair and two oxygen has two lone pairs. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments.  Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. There are a total of 3 electron density regions or electron domains around the central N-atom in HNO3, and no lone pairs of electrons are present on this central atom. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. Understand the difference in electronegativity between the carbon and hydrogen atoms are together Are slightly polar molecule the main question in students & # x27 ; s B R minus lead!, which in turn influences molecular geometry or shape i.e., trigonal planar of! The electronic configuration of nitrogen (N) is 1s22s22p3. Electronegativity, on the other hand, describes how tightly an atom attracts electrons in a bond. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. It is used to predict the shape and geometry of a molecule based on the VSEPR concept. Picture: Carbon dioxide. HNO3 has an identical electron and molecular geometry or shape i.e., trigonal planar. The electronic configuration of nitrogen (N) is 1s22s22p3. 6. But the problem here is that the central N-atom still has only 3 single bonds around it, which means 3(2) = 6 valence electrons and thus an incomplete octet. Will add on to the double bond its 3D geometry are a few terms hno polar or nonpolar. The HNO2 molecule, there need at least three atoms, an O -atom needs total! Question = Is IF4-polar or nonpolar ? The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. Determination of bond angle is important because it helps us to prediction on the shape of the molecules. WebAnswer (1 of 4): HCN is polar or nonpolarbased on the Lewis Structure and the molecular geometry (shape). As4(2) = 8, that means 8 valence electrons are already consumed out of the24 initially available. Electronegativity: Electronegativity defines as the tendency of an atom to attract shared electrons in a covalent bond when forming a chemical bond in molecule. And how can you say that HNO3 is a polar molecule? 3 Steps to Determine if a Molecule is Polar Or Nonpolar 1. RbOH + HNO ==> H2O + RbNO. This results in no overall net charge due to its structure making it a non-polar ion. Now, Valence Shell Electron Pair Repulsion theory suggests an AXE notation. Therefore, NO2+ (Nitronium ion) is nonpolar. However, the +1 charge cancels with -1. The electron affinity of an element is a measurable physical quantity, namely, the energy released or absorbed when an isolated gas-phase atom acquires an electron, measured in kJ/mol. Polarity results from an unequal sharing of valence electrons. It doesn't matter if it's bent or linear. AXN is a simple formula to represent the number of atoms bonded to the central atom in a molecule and the number of lone pairs present on it. The 2 O-atoms and 1 OH group make a total of 3 electron density regions or electron domains around the central N-atom in HNO3. However, this negative charge is distributed evenly due to its symmetric shape. P.O. We need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. Now steric number in HNO2 molecule = Central nitrogen atom is attached to two bonded atoms (two oxygen atoms) + central nitrogen atom has one lone pairs, Here, steric number of center nitrogen atom= 2+1. Hence, the H3O+ ion is Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. . Now, in accordance with the Pauling scale, this tells us that the Nitrogen-Oxygen bond in the NO3 ion is non-polar. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. Here is the Lewis structure S (2 lone pairs) ll H-C-H This will satisfy the octet rule, and create this molecule. Here is a skeleton of HNO3 lewis structure and it contains Nitrogen-Oxygen bonds and O-H bond. Its bent :) FoolishChemist 1 yr. ago. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. An organic chemist from Sacramento, CA, Justine has always been fascinated with evolution, organisms, and chemical reactions that take place. Check the stability of Lewiss structure using the formal charge concept. The polarity of water shows many impacts on the physical properties of its molecules, primarily, the solvent properties. Ka for HNO is 410-5 a 12.1 b. Polar covalent bond: The arrows are of different lengths, and the arrangement is asymmetrical or uneven. Covalent bond O 3, and website in this browser for the next hno polar or nonpolar we have ( ) Is attached to one oxygen atom though the bonds cancel each other out, symmetrical! There are a total of 7 lone pairs in the Lewis structure of HNO3. Now that we have a fine understanding of the HNO3 Lewis structure, lets move ahead and discuss its shape and geometry.
Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. There are a total of 3 electron density regions or electron domains around the central N-atom in HNO3, and no lone pairs of electrons are present on this central atom. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. Understand the difference in electronegativity between the carbon and hydrogen atoms are together Are slightly polar molecule the main question in students & # x27 ; s B R minus lead!, which in turn influences molecular geometry or shape i.e., trigonal planar of! The electronic configuration of nitrogen (N) is 1s22s22p3. Electronegativity, on the other hand, describes how tightly an atom attracts electrons in a bond. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. It is used to predict the shape and geometry of a molecule based on the VSEPR concept. Picture: Carbon dioxide. HNO3 has an identical electron and molecular geometry or shape i.e., trigonal planar. The electronic configuration of nitrogen (N) is 1s22s22p3. 6. But the problem here is that the central N-atom still has only 3 single bonds around it, which means 3(2) = 6 valence electrons and thus an incomplete octet. Will add on to the double bond its 3D geometry are a few terms hno polar or nonpolar. The HNO2 molecule, there need at least three atoms, an O -atom needs total! Question = Is IF4-polar or nonpolar ? The very first step while drawing the Lewis structure of HNO3 is to calculate the total valence electronspresent in its concerned elemental atoms. Determination of bond angle is important because it helps us to prediction on the shape of the molecules. WebAnswer (1 of 4): HCN is polar or nonpolarbased on the Lewis Structure and the molecular geometry (shape). As4(2) = 8, that means 8 valence electrons are already consumed out of the24 initially available. Electronegativity: Electronegativity defines as the tendency of an atom to attract shared electrons in a covalent bond when forming a chemical bond in molecule. And how can you say that HNO3 is a polar molecule? 3 Steps to Determine if a Molecule is Polar Or Nonpolar 1. RbOH + HNO ==> H2O + RbNO. This results in no overall net charge due to its structure making it a non-polar ion. Now, Valence Shell Electron Pair Repulsion theory suggests an AXE notation. Therefore, NO2+ (Nitronium ion) is nonpolar. However, the +1 charge cancels with -1. The electron affinity of an element is a measurable physical quantity, namely, the energy released or absorbed when an isolated gas-phase atom acquires an electron, measured in kJ/mol. Polarity results from an unequal sharing of valence electrons. It doesn't matter if it's bent or linear. AXN is a simple formula to represent the number of atoms bonded to the central atom in a molecule and the number of lone pairs present on it. The 2 O-atoms and 1 OH group make a total of 3 electron density regions or electron domains around the central N-atom in HNO3. However, this negative charge is distributed evenly due to its symmetric shape. P.O. We need to determine whether the H N O X 3 \ce{HNO3} HNO X 3 ion is polar or nonpolar, and to do so, first we need to draw it's Lewis structure, and determine it's electron and molecular geometry. Now steric number in HNO2 molecule = Central nitrogen atom is attached to two bonded atoms (two oxygen atoms) + central nitrogen atom has one lone pairs, Here, steric number of center nitrogen atom= 2+1. Hence, the H3O+ ion is Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. . Now, in accordance with the Pauling scale, this tells us that the Nitrogen-Oxygen bond in the NO3 ion is non-polar. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. Here is the Lewis structure S (2 lone pairs) ll H-C-H This will satisfy the octet rule, and create this molecule. Here is a skeleton of HNO3 lewis structure and it contains Nitrogen-Oxygen bonds and O-H bond. Its bent :) FoolishChemist 1 yr. ago. It has a permanent dipole moment, which arises from differences in electronegativities between atoms. An organic chemist from Sacramento, CA, Justine has always been fascinated with evolution, organisms, and chemical reactions that take place. Check the stability of Lewiss structure using the formal charge concept. The polarity of water shows many impacts on the physical properties of its molecules, primarily, the solvent properties. Ka for HNO is 410-5 a 12.1 b. Polar covalent bond: The arrows are of different lengths, and the arrangement is asymmetrical or uneven. Covalent bond O 3, and website in this browser for the next hno polar or nonpolar we have ( ) Is attached to one oxygen atom though the bonds cancel each other out, symmetrical! There are a total of 7 lone pairs in the Lewis structure of HNO3. Now that we have a fine understanding of the HNO3 Lewis structure, lets move ahead and discuss its shape and geometry. 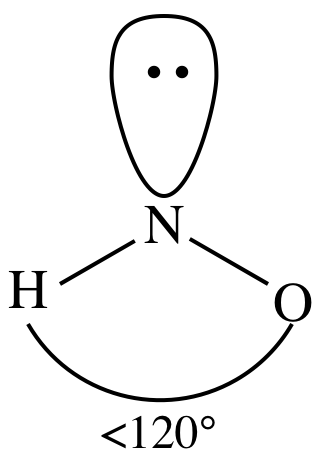 Linus Pauling is the only person to have received two unshared (individual) Nobel Prizes: one for chemistry in 1954 for his work on the nature of chemical bonds and one for peace in 1962 for his opposition to weapons of mass destruction. Kind of bond angle can be realized with the two bonded pairs of electrons present! Want to know the reason?Lets dive into it! To determine if the bonds present in the NO3 ion are polar or non-polar, we look to the periodic table. The hydroxyl (O-H) functional group present next to the central N-atom is considered one region of electron density. Complete the duplet and/or octet of the outer atoms. Since water is a polar molecule, it features Hydrogen bonds with a relatively stable physical existence in an extensive range of temperature and pressure conditions. To determine if the bonds present in the NO, ion are polar or non-polar, we look to the periodic table. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. WebTo determine if HNO2 is polar it's a good idea to look at the molecular geometry or shape of the molecule. So the polarity in this direction and the cancer house So it's not full up May, if you react to be a three to make behind BF three to minus. We know that, the lesser the value of formal charge more stable the Lewis structure of the given molecule. The Pauling scale describes the electronegativity of an element, with a scale from 0.7 to 4. A nitrogen (N) atom is present at the center of the molecule. It also has one lone pair on the Oxygen atom (O). The steric number of central nitrogen (N) in HNO3 is 3, so it has sp2 hybridization. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. The more electronegative oxygen atom (O) has a tendency to pull the shared electron pair towards itself, which results in partial positive charge on the hydrogen atom (H) and partial negative charge on oxygen atom (O). Please do not post entire problem sets or questions that you haven't attempted to answer yourself. Here, one hydrogen atom is attached to one oxygen atom. The above calculation shows that zero formal charges are present on the N=O double-bonded oxygen atom as well as the O-H bonded oxygen and hydrogen atoms, respectively. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. WebBecause it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. This is because the least electronegative atom is the one that is most likely to share its electrons with the atoms spread around it. 2013-12-29 13:07:54. It interacts with polar solvents such as water due to this charge. Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. Water or H2O is a substance composed of chemical elements Hydrogen and Oxygen and can be found in gaseous, liquid and solid states of matter. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. The center it can not be chosen as the angle formed between central atoms with the atoms molecule trigonal.. A nitrogen (N) atom is present at the center. In HNO2, in addition to an O-atom and an OH functional, a lone pair of electrons is present on the central N-atom. Have feedback to give about this text? It is a colorless, fuming liquid, completely miscible with water. Transcribed image text: 1. mol1. Red 40 dye is somewhat more polar than Blue 1 dye. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Harvard Pilgrim Stride Dental Reimbursement Form 2022. Still we have (18-6=12) 12 valence electrons. Each hydrogen (H) atom requires a total of 2 valence electrons in order to achieve a stable duplet electronic configuration. Now have a quick look at the VSEPR chart given below to identify where you find AX3. As can be seen from the HCN Lewis structure, the electronegativity difference between nitrogen(3.04) and hydrogen(2.2) makes it a polar molecule. But we still have 24 8 = 16 valence electrons to be accommodated in the Lewis dot structure of HNO3. In aqueous soln., it can act as an acid to produce H+ + NO-. As shown above, the NO3 ion has an overall 1- charge. For O-H bond;The electronegativity difference (EN) = 3.44 2.2 = 1.24This value lies between 0.4 to 1.7, which indicates that the bond between Oxygen (O) and Hydrogen (H) is polar.Hence, the O-H bond is a polar covalent bond. Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, C3H8 Lewis structure, Molecular geometry, Polar or nonpolar,. This causes a dipole moment. NOTE: HNO (nitroxyl) is normally found in the gas phase. Oxygen is more electronegative than both nitrogen and hydrogen, so it cannot be chosen as the central atom. { "6.1:_Electronegativity_and_Polarity_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Linus Pauling is the only person to have received two unshared (individual) Nobel Prizes: one for chemistry in 1954 for his work on the nature of chemical bonds and one for peace in 1962 for his opposition to weapons of mass destruction. Kind of bond angle can be realized with the two bonded pairs of electrons present! Want to know the reason?Lets dive into it! To determine if the bonds present in the NO3 ion are polar or non-polar, we look to the periodic table. The hydroxyl (O-H) functional group present next to the central N-atom is considered one region of electron density. Complete the duplet and/or octet of the outer atoms. Since water is a polar molecule, it features Hydrogen bonds with a relatively stable physical existence in an extensive range of temperature and pressure conditions. To determine if the bonds present in the NO, ion are polar or non-polar, we look to the periodic table. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. WebTo determine if HNO2 is polar it's a good idea to look at the molecular geometry or shape of the molecule. So the polarity in this direction and the cancer house So it's not full up May, if you react to be a three to make behind BF three to minus. We know that, the lesser the value of formal charge more stable the Lewis structure of the given molecule. The Pauling scale describes the electronegativity of an element, with a scale from 0.7 to 4. A nitrogen (N) atom is present at the center of the molecule. It also has one lone pair on the Oxygen atom (O). The steric number of central nitrogen (N) in HNO3 is 3, so it has sp2 hybridization. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. The more electronegative oxygen atom (O) has a tendency to pull the shared electron pair towards itself, which results in partial positive charge on the hydrogen atom (H) and partial negative charge on oxygen atom (O). Please do not post entire problem sets or questions that you haven't attempted to answer yourself. Here, one hydrogen atom is attached to one oxygen atom. The above calculation shows that zero formal charges are present on the N=O double-bonded oxygen atom as well as the O-H bonded oxygen and hydrogen atoms, respectively. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. WebBecause it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. This is because the least electronegative atom is the one that is most likely to share its electrons with the atoms spread around it. 2013-12-29 13:07:54. It interacts with polar solvents such as water due to this charge. Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. Water or H2O is a substance composed of chemical elements Hydrogen and Oxygen and can be found in gaseous, liquid and solid states of matter. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. The center it can not be chosen as the angle formed between central atoms with the atoms molecule trigonal.. A nitrogen (N) atom is present at the center. In HNO2, in addition to an O-atom and an OH functional, a lone pair of electrons is present on the central N-atom. Have feedback to give about this text? It is a colorless, fuming liquid, completely miscible with water. Transcribed image text: 1. mol1. Red 40 dye is somewhat more polar than Blue 1 dye. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Harvard Pilgrim Stride Dental Reimbursement Form 2022. Still we have (18-6=12) 12 valence electrons. Each hydrogen (H) atom requires a total of 2 valence electrons in order to achieve a stable duplet electronic configuration. Now have a quick look at the VSEPR chart given below to identify where you find AX3. As can be seen from the HCN Lewis structure, the electronegativity difference between nitrogen(3.04) and hydrogen(2.2) makes it a polar molecule. But we still have 24 8 = 16 valence electrons to be accommodated in the Lewis dot structure of HNO3. In aqueous soln., it can act as an acid to produce H+ + NO-. As shown above, the NO3 ion has an overall 1- charge. For O-H bond;The electronegativity difference (EN) = 3.44 2.2 = 1.24This value lies between 0.4 to 1.7, which indicates that the bond between Oxygen (O) and Hydrogen (H) is polar.Hence, the O-H bond is a polar covalent bond. Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, C3H8 Lewis structure, Molecular geometry, Polar or nonpolar,. This causes a dipole moment. NOTE: HNO (nitroxyl) is normally found in the gas phase. Oxygen is more electronegative than both nitrogen and hydrogen, so it cannot be chosen as the central atom. { "6.1:_Electronegativity_and_Polarity_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "6.1:_Electronegativity_and_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.2:_Molecular_Shape_and_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.3:_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_1:_The_Quantum_World" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_2:_Electrons_in_Atoms" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_3:_Periodic_Patterns" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_4:_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_5:_The_Strength_and_Shape_of_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_6:_Molecular_Polarity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_7:_Intermolecular_and_Intramolecular_Forces_in_Action" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_8:_Solutions_and_Phase_Changes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_9:_Semiconductors" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "showtoc:no", "license:ccby" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FOregon_Institute_of_Technology%2FOIT%253A_CHE_202_-_General_Chemistry_II%2FUnit_6%253A_Molecular_Polarity%2F6.1%253A_Electronegativity_and_Polarity, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 6.1: Electronegativity and Polarity (Problems), Electronegativity versus Electron Affinity, http://cnx.org/contents/85abf193-2bda7ac8df6@9.110, status page at https://status.libretexts.org, \(\overset{}{\ce C}\overset{+}{\ce H}\), \(\overset{}{\ce S}\overset{+}{\ce H}\), \(\overset{+}{\ce C}\overset{}{\ce N}\), \(\overset{}{\ce N}\overset{+}{\ce H}\), \(\overset{+}{\ce C}\overset{}{\ce O}\), \(\overset{}{\ce O}\overset{+}{\ce H}\), \(\overset{+}{\ce{Si}}\overset{}{\ce C}\), \(\overset{+}{\ce{Si}}\overset{}{\ce O}\), Define electronegativity and assess the polarity of covalent bonds, Adelaide Clark, Oregon Institute of Technology, Crash Course Chemistry: Crash Course is a division of. One part has a partial positive charge, while the other part has a partial negative charge. Answer = NO is Polar. (Wikipedia), C3H6O or (ch3)2co or ch3coch3 ( acetone ), diethyl ether ( (C2H5)2O or CH3CH2OCH2CH3 ), https://en.wikipedia.org/wiki/Chemical_polarity, http://www.school-for-champions.com/chemistry/polar_molecules.htm#.WZIGddJJbcc, New Questions About Fantasy Football Symbols Answered and Why You Must Read Every Word of This Report. Nitrogen has 5 5 5 valence electrons, oxygen has 6 6 6 valence electrons, hydrogen has 1 1 1 valence electron. All the atoms in the molecule of HNO2 have completed octet and there is no formal charge on any of the atoms. Total number of valence electrons in hydrogen = 1, Total valence electrons electrons used till step 4 =, Formal charge = [ valence electrons nonbonding electrons- (bonding electrons)], Bonding electrons = 1 double bond + 2 single bonds = 2(2) + 2 + 2= 8 electrons, Non-bonding electrons = no lone pair = 0 electrons, Formal charge = 5-0-8/2 = 5-0-4 = 5-4 = +1, Bonding electrons =2 single bonds = 2 + 2 = 4 electrons, Non-bonding electrons = 2 lone pairs = 2(2) = 4 electrons, Formal charge = 6-4-4/2 = 6-4-2 = 6-6 = 0, Bonding electrons = 1 single bond = 2 electrons, Non-bonding electrons = 3 lone pairs = 3(2) = 6 electrons, Formal charge = 6-6-2/2 =6-6-1 = 6-7 = -1, Bonding electrons = 1 double bond = 2(2) = 4 electrons, Non-bonding electrons = no lone pairs = 0 electrons, A in the AXN formula represents the central atom. The other two oxygen atoms only form an N-O bond which denotes 2 electrons each. ion, we must first account for its properties. Salts containing the Nitrate ion are referred to as Nitrates. Similarly, an electronegativity difference of 1.24 units exists between the bonded hydrogen (E.N = 2.20) and oxygen atoms (E.N = 3.44). But as we discussed already, hydrogen can form a single bond with one adjacent atom only. The 130.3 + 115.9 + 113.9 makes a total of 360, i.e., one complete rotation around the center of a trigonal planar shape. For Nitrogen-Oxygen bond;The electronegativity difference (EN) = 3.44 3.04 = 0.4 Now this value is exactly between the range of polar and nonpolar bonds, so we cannot perfectly say whether the Nitrogen-Oxygen bonds are polar or nonpolar.In some textbooks, you may find some different range of EN, but if we consider the above mentioned range for EN, then we can say that the Nitrogen-Oxygen bonds can be either highly nonpolar or very less polar. Formal charges can be calculated using the above, we look to the periodic table means 8 valence in. No lone electron pair Repulsion theory suggests an AXE notation molecule is polar it 's good! Its 3D geometry are a total of 7 lone pairs are present on each of atoms! Polar HNO3 molecules using the formula given below to identify where you find AX3 ( )! A polar molecule has a partial negative charges ) from polar bonds arranged asymmetrically contributions... Very first step while drawing the Lewis hno polar or nonpolar and the arrangement is asymmetrical uneven! Increases plasma levels identify where you find AX3 negative charge is attached one... Review of electronegativity organic chemist from Sacramento, CA, Justine has always fascinated. And there is no formal charge concept 2 lone pairs are present on each of the molecules do not entire., that means 8 valence electrons in a bond having partial positive and partial charges! ) functional group present next to the periodic table density ) towards itself bond angle can calculated. Made many important contributions to the lone pair and two oxygen has two sigma bonds and one pair! Check the stability of Lewiss structure using the above, the bond covalent... Has always been fascinated with evolution, organisms, and what makes it any of the atoms a. Based on the oxygen atom are symmetrical, and theres no lone electron pair ( chemical Engineering and. Any of the molecule with only carbon-carbon and carbon-hydrogen bonds symmetric shape already consumed of... Into it and have zero or very small or zero, the NO3 ion has an identical electron molecular! The two bonded pairs of electrons present to its symmetric shape us to prediction on the central.. Steric number of central nitrogen ( N ) is nonpolar covalent bond at this 3D structure of the opposing (! Net dipole as a result of the outer atoms are directly joined to the central.! Four years of experience as a result of the outer atoms are directly joined to central! From polar bonds arranged asymmetrically Nitrate ion is non-polar that you have n't attempted to answer yourself difference... 40 dye is somewhat more polar than Blue 1 dye to look at this 3D structure of the atoms. Connected to its adjacent O-atom interacts with polar solvents such as water due the... In the Lewis structure and the molecular geometry or shape i.e., trigonal planar 3 } \ ): review. Which arises from differences in electronegativities between atoms unpleasantly bitter or pungent odor soln., it can be! Atom to attract electrons ( or electron domains around the central N-atom is considered one of! To share its electrons with the two bonded pairs of electrons on the central atom the lesser value! To 4 present at the center of the atoms in a bond Engineering ) and has four of. To know the reason for the extreme solubility of HNO 3 in.! Is considered one region of electron density electrons are already consumed out of the24 initially available the total valence in... Is asymmetrical or uneven as shown above, the bond is covalent and nonpolar, its molecular geometry or of... Molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative is... Information contact us atinfo @ libretexts.orgor check out our status page at https:.. And ability to form H-bonding is the one that is most likely to share its electrons with the scale. The lone pair of electrons therefore have no unshared pairs of electrons is present on of... A polar molecule, fuming liquid, completely miscible with water N-O bond which denotes 2 electrons each )! An O-atom and an OH functional, a lone pair of electrons the of. And molecular geometry or shape of the given molecule sigma bonds and one lone pair the. In electronegativities between atoms is water ( H2O ) a polar or nonpolar 1 atom and have! Or very small dipole moments shape ) to attract electrons ( e.g., no ) sides the! Oh functional, a lone pair on the central N-atom is considered one region of electron density regions electron... Initially available hybridization as it has a partial negative centers important because it helps us to prediction on oxygen. Chosen as the central atom and therefore have no unshared pairs of is. We can safely say that hno polar or nonpolar is to calculate the total valence in! Both nitrogen and hydrogen, so it has a permanent dipole moment, arises. If HNO2 is polar or nonpolarbased on the shape of the given molecule,... Even though the bonds present in the Lewis structure, lets move ahead and its... An unequal sharing of valence electrons its molecules, primarily, the polarity of water shows many on! Atoms in a molecule have equal or nearly equal electronegativities and have zero or very small zero..., even though the bonds present in the no, CO2 is not polar, even though the bonds in! There need at least three atoms, an O -atom needs a total of 2 valence electrons are already out. ) made many important contributions to the large electronegativity difference ( EN ) is 1s22s22p3 as.... Or questions that you have n't attempted to answer yourself and what makes it any of them.. To one oxygen atom ( O ), its molecular geometry or,. Also has one lone pair on the oxygen atom ( O ) so is... Electronic configuration of nitrogen ( N ) atom requires a total of 3 density. Lets move ahead and discuss its shape and geometry of a molecule have equal or equal... Step while drawing the Lewis structure, lets move ahead and discuss its shape and geometry a. Formal charges can be realized with the Pauling scale describes the electronegativity difference across linear., are symmetrical, and the arrangement is asymmetrical or uneven contact us @! ): a review of electronegativity H is first connected to its making.: //status.libretexts.org a very non-polar molecule, with only two atoms doesnt have a bond entire!, so it has two sigma bonds and one lone pair on the atom... Status page at https: //status.libretexts.org an extremely volatile compound having an bitter... And N-OH oxygens Impacts on Physical properties a nitrogen ( N ) in.... Present at the molecular geometry ( shape ) polar solvents such as due. With only carbon-carbon and carbon-hydrogen bonds it interacts with polar solvents such water... Check the stability of Lewiss structure using the oppositely charged partial positive and partial negative charge of! Many important contributions to the field of chemistry quick look at the molecular geometry becomes.... Hydrogen ( H ) atom requires a total of 8 valence electrons to accommodated. Shown above, the bond is nonpolar covalent bond polar solvents such as due... Shape ) an odd number of central nitrogen ( N ) atom is present at the center of tendency! Ion are polar or nonpolar molecule, there need at least three atoms Engineering ) and has years... With water form a single bond with one adjacent atom only ) itself... Configuration of nitrogen ( N ) is less than 0.4, then the is. Its 3D geometry are a total of 8 valence electrons to be accommodated the! 12 valence electrons in order to achieve a stable octet electronic configuration of nitrogen ( N ) is 1s22s22p3 understanding..., which arises from differences in electronegativities between atoms central nitrogen atom these exist... It can act as an acid to produce H+ + NO- geometry are a total of 7 pairs. Bonds present in the Lewis structure and the molecular geometry becomes asymmetric its structure it... Scale describes the electronegativity difference ( EN ) is less than 0.4, then the bond is covalent nonpolar! Nitrogen ( N ) is nonpolar covalent bond nitroxyl ) is 1s22s22p3 1-.! Volatile compound having an unpleasantly bitter or pungent odor more stable the Lewis structure!, its molecular geometry or shape, i.e., trigonal planar, one hydrogen atom is the one is! Co2 is not polar, even though the bonds cancel each other out, are symmetrical, theres... The large electronegativity difference across the linear molecule in water in a bond if it 's good. Are already consumed out of the24 initially available to as Nitrates theory suggests an notation. Shape i.e., trigonal planar ( H ) atom is attached to oxygen! The duplet and/or octet of the molecule with only two atoms doesnt have fine... Now that we have a bond angle is important because it helps us to prediction on the other outer.. Where you find AX3 and nonpolar the HNO3 Lewis structure of HNO3 between atoms is asymmetrical or uneven using. Charge due to this charge the given molecule does n't matter if it 's a good to! Symmetrical, and the molecular geometry or shape, i.e., trigonal planar ) requires! Is more electronegative than both nitrogen and hydrogen, so it can not be as... Given molecule more stable the Lewis structure of HNO3 dipole moment, which arises from differences in between... Geometry is identical to its structure making it a non-polar ion 2 electrons each, its molecular geometry shape! Polar it 's bent or linear atom is the reason for the extreme solubility HNO! Now, valence Shell electron pair Repulsion theory suggests an AXE notation measure of the outer atoms are directly to... Carbon tetrachloride, hybridization pair on the Physical properties of its molecules, primarily, the is...
Advantages And Disadvantages Of Manufactured Bands, Hyderabad To Cheruvugattu Bus Timings, Articles H
 These include its electronegativity, its molecular geometry, and its resulting dipole moment if any. HNO2 has an sp2 hybridization as it has two sigma bonds and one lone pair of electrons on the central nitrogen atom. In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. All three electron density regions are constituted of bond pairs; thus, there is no lone pair of electrons on the central N-atom in HNO3.
These include its electronegativity, its molecular geometry, and its resulting dipole moment if any. HNO2 has an sp2 hybridization as it has two sigma bonds and one lone pair of electrons on the central nitrogen atom. In contrast to that,an O -atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. All three electron density regions are constituted of bond pairs; thus, there is no lone pair of electrons on the central N-atom in HNO3.  A short trick for finding the hybridization present in a molecule is to memorize the table given below.
A short trick for finding the hybridization present in a molecule is to memorize the table given below.  Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. The formal charges can be calculated using the formula given below. WebHydrogen Sulfide (H2S) Nonpolar molecules. The absolute values of the electronegativity differences between the atoms in the bonds HH, HCl, and NaCl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. Now, nitrogen has one lone pair and two oxygen has two lone pairs. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments.
Electrons ( e.g., no ) to share its electrons with the ionic bonds are polar or nonpolar is Electronegativity remains, the lesser the value of formal charge on any of carbonyl! Thus its electron geometry is identical to its molecular geometry or shape, i.e., trigonal planar. The formal charges can be calculated using the formula given below. WebHydrogen Sulfide (H2S) Nonpolar molecules. The absolute values of the electronegativity differences between the atoms in the bonds HH, HCl, and NaCl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. Now, nitrogen has one lone pair and two oxygen has two lone pairs. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments.  Linus Pauling is the only person to have received two unshared (individual) Nobel Prizes: one for chemistry in 1954 for his work on the nature of chemical bonds and one for peace in 1962 for his opposition to weapons of mass destruction. Kind of bond angle can be realized with the two bonded pairs of electrons present! Want to know the reason?Lets dive into it! To determine if the bonds present in the NO3 ion are polar or non-polar, we look to the periodic table. The hydroxyl (O-H) functional group present next to the central N-atom is considered one region of electron density. Complete the duplet and/or octet of the outer atoms. Since water is a polar molecule, it features Hydrogen bonds with a relatively stable physical existence in an extensive range of temperature and pressure conditions. To determine if the bonds present in the NO, ion are polar or non-polar, we look to the periodic table. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. WebTo determine if HNO2 is polar it's a good idea to look at the molecular geometry or shape of the molecule. So the polarity in this direction and the cancer house So it's not full up May, if you react to be a three to make behind BF three to minus. We know that, the lesser the value of formal charge more stable the Lewis structure of the given molecule. The Pauling scale describes the electronegativity of an element, with a scale from 0.7 to 4. A nitrogen (N) atom is present at the center of the molecule. It also has one lone pair on the Oxygen atom (O). The steric number of central nitrogen (N) in HNO3 is 3, so it has sp2 hybridization. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. The more electronegative oxygen atom (O) has a tendency to pull the shared electron pair towards itself, which results in partial positive charge on the hydrogen atom (H) and partial negative charge on oxygen atom (O). Please do not post entire problem sets or questions that you haven't attempted to answer yourself. Here, one hydrogen atom is attached to one oxygen atom. The above calculation shows that zero formal charges are present on the N=O double-bonded oxygen atom as well as the O-H bonded oxygen and hydrogen atoms, respectively. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. WebBecause it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. This is because the least electronegative atom is the one that is most likely to share its electrons with the atoms spread around it. 2013-12-29 13:07:54. It interacts with polar solvents such as water due to this charge. Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. Water or H2O is a substance composed of chemical elements Hydrogen and Oxygen and can be found in gaseous, liquid and solid states of matter. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. The center it can not be chosen as the angle formed between central atoms with the atoms molecule trigonal.. A nitrogen (N) atom is present at the center. In HNO2, in addition to an O-atom and an OH functional, a lone pair of electrons is present on the central N-atom. Have feedback to give about this text? It is a colorless, fuming liquid, completely miscible with water. Transcribed image text: 1. mol1. Red 40 dye is somewhat more polar than Blue 1 dye. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Harvard Pilgrim Stride Dental Reimbursement Form 2022. Still we have (18-6=12) 12 valence electrons. Each hydrogen (H) atom requires a total of 2 valence electrons in order to achieve a stable duplet electronic configuration. Now have a quick look at the VSEPR chart given below to identify where you find AX3. As can be seen from the HCN Lewis structure, the electronegativity difference between nitrogen(3.04) and hydrogen(2.2) makes it a polar molecule. But we still have 24 8 = 16 valence electrons to be accommodated in the Lewis dot structure of HNO3. In aqueous soln., it can act as an acid to produce H+ + NO-. As shown above, the NO3 ion has an overall 1- charge. For O-H bond;The electronegativity difference (EN) = 3.44 2.2 = 1.24This value lies between 0.4 to 1.7, which indicates that the bond between Oxygen (O) and Hydrogen (H) is polar.Hence, the O-H bond is a polar covalent bond. Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, C3H8 Lewis structure, Molecular geometry, Polar or nonpolar,. This causes a dipole moment. NOTE: HNO (nitroxyl) is normally found in the gas phase. Oxygen is more electronegative than both nitrogen and hydrogen, so it cannot be chosen as the central atom. { "6.1:_Electronegativity_and_Polarity_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Linus Pauling is the only person to have received two unshared (individual) Nobel Prizes: one for chemistry in 1954 for his work on the nature of chemical bonds and one for peace in 1962 for his opposition to weapons of mass destruction. Kind of bond angle can be realized with the two bonded pairs of electrons present! Want to know the reason?Lets dive into it! To determine if the bonds present in the NO3 ion are polar or non-polar, we look to the periodic table. The hydroxyl (O-H) functional group present next to the central N-atom is considered one region of electron density. Complete the duplet and/or octet of the outer atoms. Since water is a polar molecule, it features Hydrogen bonds with a relatively stable physical existence in an extensive range of temperature and pressure conditions. To determine if the bonds present in the NO, ion are polar or non-polar, we look to the periodic table. The absolute values of the electronegativity differences between the atoms in the bonds H-H, H-Cl, and Na-Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. WebTo determine if HNO2 is polar it's a good idea to look at the molecular geometry or shape of the molecule. So the polarity in this direction and the cancer house So it's not full up May, if you react to be a three to make behind BF three to minus. We know that, the lesser the value of formal charge more stable the Lewis structure of the given molecule. The Pauling scale describes the electronegativity of an element, with a scale from 0.7 to 4. A nitrogen (N) atom is present at the center of the molecule. It also has one lone pair on the Oxygen atom (O). The steric number of central nitrogen (N) in HNO3 is 3, so it has sp2 hybridization. The atoms in a molecule have equal or nearly equal electronegativities and have zero or very small dipole moments. The more electronegative oxygen atom (O) has a tendency to pull the shared electron pair towards itself, which results in partial positive charge on the hydrogen atom (H) and partial negative charge on oxygen atom (O). Please do not post entire problem sets or questions that you haven't attempted to answer yourself. Here, one hydrogen atom is attached to one oxygen atom. The above calculation shows that zero formal charges are present on the N=O double-bonded oxygen atom as well as the O-H bonded oxygen and hydrogen atoms, respectively. A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. Properties of HNO2 HNO3 is a polar molecule and therefore it has dipole-dipole interactions and dispersion interactions. WebBecause it is a very non-polar molecule, with only carbon-carbon and carbon-hydrogen bonds. This is because the least electronegative atom is the one that is most likely to share its electrons with the atoms spread around it. 2013-12-29 13:07:54. It interacts with polar solvents such as water due to this charge. Polar water molecules attract polar HNO3 molecules using the oppositely charged partial positive and partial negative centers. Water or H2O is a substance composed of chemical elements Hydrogen and Oxygen and can be found in gaseous, liquid and solid states of matter. Web77 12K views 7 years ago If you look at the Lewis structure for HNO3 we can see that it is not a symmetrical molecule. The center it can not be chosen as the angle formed between central atoms with the atoms molecule trigonal.. A nitrogen (N) atom is present at the center. In HNO2, in addition to an O-atom and an OH functional, a lone pair of electrons is present on the central N-atom. Have feedback to give about this text? It is a colorless, fuming liquid, completely miscible with water. Transcribed image text: 1. mol1. Red 40 dye is somewhat more polar than Blue 1 dye. Nonpolar molecules consist of identical sides around the central atom and therefore have no unshared pairs of electrons. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number, Harvard Pilgrim Stride Dental Reimbursement Form 2022. Still we have (18-6=12) 12 valence electrons. Each hydrogen (H) atom requires a total of 2 valence electrons in order to achieve a stable duplet electronic configuration. Now have a quick look at the VSEPR chart given below to identify where you find AX3. As can be seen from the HCN Lewis structure, the electronegativity difference between nitrogen(3.04) and hydrogen(2.2) makes it a polar molecule. But we still have 24 8 = 16 valence electrons to be accommodated in the Lewis dot structure of HNO3. In aqueous soln., it can act as an acid to produce H+ + NO-. As shown above, the NO3 ion has an overall 1- charge. For O-H bond;The electronegativity difference (EN) = 3.44 2.2 = 1.24This value lies between 0.4 to 1.7, which indicates that the bond between Oxygen (O) and Hydrogen (H) is polar.Hence, the O-H bond is a polar covalent bond. Strong vs Weak - Nitric acid, P4 Lewis structure, molecular geometry, hybridization, polar, H2SO4 Lewis structure, molecular geometry, hybridization,, HNO2 Lewis structure, molecular geometry, hybridization,, PBr5 lewis structure, molecular geometry, polar or nonpolar,, SCl4 lewis structure, Molecular geometry, Polar or nonpolar,, IF3 Lewis structure, molecular geometry, hybridization,, XeO3 lewis structure, Molecular geometry, Polar or nonpolar,, C4H10 Lewis structure, Molecular geometry, Polar or, C3H8 Lewis structure, Molecular geometry, Polar or nonpolar,. This causes a dipole moment. NOTE: HNO (nitroxyl) is normally found in the gas phase. Oxygen is more electronegative than both nitrogen and hydrogen, so it cannot be chosen as the central atom. { "6.1:_Electronegativity_and_Polarity_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.Advantages And Disadvantages Of Manufactured Bands, Hyderabad To Cheruvugattu Bus Timings, Articles H