lead bromide electrolysis equation
 Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific.
Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. 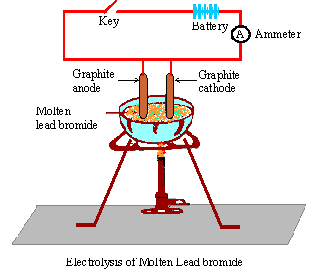 Name : A non-metallic element which is a conductor of electricity. If you are determined to make Calcium metal it might be worth electrolysing an aqueous solution of C a C l X 2 to evolve the C l X 2 ( g) (do this responsibly outside/in fume hood of course as C l X 2 ( g) is poisonous.) Cathode : H+ + e- [H] 2 [H] H2 OH- - e- OH Anode : 4OH 2H2O + O2 5. Hence, ions become mobile. What pressure is to be expected in the test section if the atmospheric temperature and pressure are: If HX is a weak acid, what particles will be present in its dilute solution apart from those of water? Choose the correct answer from the option given below:In electrolysis of molten lead bromine anode is made up of, Choose the correct answer from the option given below:Electrolysis of acidulated water is used in the production of. He introduced the term electrolysis in 1834. Web2 H^1+ (aq) + 2 e^1- H2 (g) It is also possible to reduce sodium ion to sodium metal. Stewart has been an enthusiastic GCSE, IGCSE, A Level and IB teacher for more than 30 years in the UK as well as overseas, and has also been an examiner for IB and A Level. Learning to name chemical compounds requires that you: For Lead (II) bromide use the hints and resources below to help write the formula. Notes - Delivery *Estimated delivery dates include seller's handling time, origin ZIP Code, destination ZIP Code and time of acceptance and will depend on shipping service selected and receipt of cleared payment. Something went wrong. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. WebThe electrolysis of molten lead bromide, PbBr 2 (note: there is no water). The apparatus is set up as shown in Figure. How is impure copper purified by electrolysis ? Balance the charges on Pb and Br by modifying the subscripts. Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. VIEW SOLUTION. (c) 20C,92kPa20^{\circ} \mathrm{C}, 92~\mathrm{kPa}20C,92kPa ? Exercise 4 | Q 6 | Page 148 Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. (A) Non-electrolyte, (B) Strong electrolyte, (C) Weak electrolyte, (D) Metallic conductor(i) Molten ionic compound(ii) Carbon tetrachloride(iii) An aluminium wire(iv) A solution containing solvent molecules, solute molecules and ions formed by the dissociation of solute molecules(v) A sugar solution with sugar molecules and water molecules. (e) Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. IBO was not involved in the production of, and does not endorse, the resources created by Save My Exams. For example, it doesn't show that there are twice as many bromide ions as there are lead ions.
Name : A non-metallic element which is a conductor of electricity. If you are determined to make Calcium metal it might be worth electrolysing an aqueous solution of C a C l X 2 to evolve the C l X 2 ( g) (do this responsibly outside/in fume hood of course as C l X 2 ( g) is poisonous.) Cathode : H+ + e- [H] 2 [H] H2 OH- - e- OH Anode : 4OH 2H2O + O2 5. Hence, ions become mobile. What pressure is to be expected in the test section if the atmospheric temperature and pressure are: If HX is a weak acid, what particles will be present in its dilute solution apart from those of water? Choose the correct answer from the option given below:In electrolysis of molten lead bromine anode is made up of, Choose the correct answer from the option given below:Electrolysis of acidulated water is used in the production of. He introduced the term electrolysis in 1834. Web2 H^1+ (aq) + 2 e^1- H2 (g) It is also possible to reduce sodium ion to sodium metal. Stewart has been an enthusiastic GCSE, IGCSE, A Level and IB teacher for more than 30 years in the UK as well as overseas, and has also been an examiner for IB and A Level. Learning to name chemical compounds requires that you: For Lead (II) bromide use the hints and resources below to help write the formula. Notes - Delivery *Estimated delivery dates include seller's handling time, origin ZIP Code, destination ZIP Code and time of acceptance and will depend on shipping service selected and receipt of cleared payment. Something went wrong. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. WebThe electrolysis of molten lead bromide, PbBr 2 (note: there is no water). The apparatus is set up as shown in Figure. How is impure copper purified by electrolysis ? Balance the charges on Pb and Br by modifying the subscripts. Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. VIEW SOLUTION. (c) 20C,92kPa20^{\circ} \mathrm{C}, 92~\mathrm{kPa}20C,92kPa ? Exercise 4 | Q 6 | Page 148 Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. (A) Non-electrolyte, (B) Strong electrolyte, (C) Weak electrolyte, (D) Metallic conductor(i) Molten ionic compound(ii) Carbon tetrachloride(iii) An aluminium wire(iv) A solution containing solvent molecules, solute molecules and ions formed by the dissociation of solute molecules(v) A sugar solution with sugar molecules and water molecules. (e) Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. IBO was not involved in the production of, and does not endorse, the resources created by Save My Exams. For example, it doesn't show that there are twice as many bromide ions as there are lead ions.  Exercise 6 | Q 4.3 | Page 117. In the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode. It has ____________ of electrons. Electrons flow from the anode to the cathode through the connecting wire in the external circuit. The electrolyte used for electroplating an article with silver is: M is a metal above hydrogen in the activity series and its oxide has the formula M2O. Web(i) The half-equations for the electrolysis of lead (II) bromide: (a) The negative cathode electrode reaction for the electrolysis of molten lead (II) bromide The positive lead (II) ions are attracted to the negative electrode and are discharged to Apparatus: Batteries, switch, carbon electrodes with holders, connecting wires with crocodile clips, ammeter, crucible, tripod stand, pipe-clay triangle, Bunsen burner, 250 cm 3 beaker and tongs. Explain the observation. Select the correct answer from the choicesa,b,c and d which are given. Give reason why: Although copper is a good conductor of electricity, it is a non-electrolyte. A solution contains magnesium ions (Mg+2), iron (II) ions (Fe+2) and copper ions (Cu+2) . WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. WebWhat are the half equations representing the changes of Pb2+ and Br- in the electrolysis of lead bromide? WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. CBr 4 + H 2 SO 4 Brown bromine gas is formed at the anode (positive electrode). Take this picture for example: http://img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif. Potassium Chloride. What should be the nature of the anode? Michael Faraday was a pioneer in the field of electrolysis. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. (c) State one condition to ensure that the deposit is smooth, firm and long lasting.
Exercise 6 | Q 4.3 | Page 117. In the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode. It has ____________ of electrons. Electrons flow from the anode to the cathode through the connecting wire in the external circuit. The electrolyte used for electroplating an article with silver is: M is a metal above hydrogen in the activity series and its oxide has the formula M2O. Web(i) The half-equations for the electrolysis of lead (II) bromide: (a) The negative cathode electrode reaction for the electrolysis of molten lead (II) bromide The positive lead (II) ions are attracted to the negative electrode and are discharged to Apparatus: Batteries, switch, carbon electrodes with holders, connecting wires with crocodile clips, ammeter, crucible, tripod stand, pipe-clay triangle, Bunsen burner, 250 cm 3 beaker and tongs. Explain the observation. Select the correct answer from the choicesa,b,c and d which are given. Give reason why: Although copper is a good conductor of electricity, it is a non-electrolyte. A solution contains magnesium ions (Mg+2), iron (II) ions (Fe+2) and copper ions (Cu+2) . WebThe rules for balancing redox equations involve adding H +, H 2 O, and OH - to one side or the other of the half-equations. WebWhat are the half equations representing the changes of Pb2+ and Br- in the electrolysis of lead bromide? WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. CBr 4 + H 2 SO 4 Brown bromine gas is formed at the anode (positive electrode). Take this picture for example: http://img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif. Potassium Chloride. What should be the nature of the anode? Michael Faraday was a pioneer in the field of electrolysis. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. (c) State one condition to ensure that the deposit is smooth, firm and long lasting.  Molten lead (II) bromide The electrolyte is molten PbBr 2. (b) Of what substance must the anode be made up of? In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. Make a neatly labeled sketch to show how a brass spoon can be plated with silver. The sodium ions are reduced to sodium atoms by gaining electrons. The following questions are about electroplating of copper wire with silver. (a) Write equations to show how X and Y form ions. Pb2+ + 2e- -> Pb 2Br- -> Br2 + 2e- Lead ions undergo reduction (gain WebFind many great new & used options and get the best deals for Kara 3D Clear File Raw Bromide at the best online prices at eBay! This option is wrong. 2. cathode (- ve).
Molten lead (II) bromide The electrolyte is molten PbBr 2. (b) Of what substance must the anode be made up of? In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. Make a neatly labeled sketch to show how a brass spoon can be plated with silver. The sodium ions are reduced to sodium atoms by gaining electrons. The following questions are about electroplating of copper wire with silver. (a) Write equations to show how X and Y form ions. Pb2+ + 2e- -> Pb 2Br- -> Br2 + 2e- Lead ions undergo reduction (gain WebFind many great new & used options and get the best deals for Kara 3D Clear File Raw Bromide at the best online prices at eBay! This option is wrong. 2. cathode (- ve).  We can show this with the help of equations at cathode and anode like: At anode: $2B{r^ - } \to B{r_2}$ At cathode: WebExplain the following observations: When lead(II) bromide is heated until it melts and an electric current passed through, a silvery coloured liquid is found under the negative electrode (cathode) and a brown gas appears at the positive electrode (anode). Stewart specialises in Chemistry, but has also taught Physics and Environmental Systems and Societies. Chloride ions lose electrons ( oxidation) to form chlorine atoms. Sodium Chloride. State your observation for the following electrolytic reaction:Solid copper sulphate is electrolysed between platinum electrodes. In the electrolysis of molten lead(II) bromide the half equation at the negative electrode (cathode) is: At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: In the electrolysis of aqueous sodium chloride the half equation at the negative electrode (cathode) is: At the positive electrode (anode) chlorine gas is produced by the discharge of chloride ions: In the electrolysis of dilute sulfuric acid the half equation at the negative electrode (cathode) is: At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: In the electrolysis of aqueous copper(II) sulfate the half equation at the negative electrode (cathode) is. The molten lead(II) bromide is carefully poured into a beaker using a pair of tongs. Its chemical formula is PbBr2. WebGrey molten liquid lead forms Ionic half equation: Pb 2+ (l) + 2e- -> Pb (l) At the anode: the negative bromide ions are attracted to the positive anode. (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? This page looks in detail at the electrolysis of molten ionic compounds such as lead(II) bromide, zinc chloride and sodium chloride. Explain how electrolysis is an example of Redox reaction. Give reason. 2Br Br 2 + 2e Anode : OH- - e- OH 4OH- 2H2O + H2 4. Connect and share knowledge within a single location that is structured and easy to search. 1. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so? The lead(II) ions are reduced to lead atoms by gaining electrons. But why do they become neutral after they reach the electrodes. So, naturally they should be attracted to the cathode and the anode respectively. Free shipping for many products! Find the odd one out from the following and explain your choice : Al(OH)3, Pb(OH)2,Mg(OH)2,Zn(OH)2. State one observation when electricity is passed through molten lead bromide. To examine associations between the pyridostigmine bromide (PB) pill and/or pesticide exposure during the 1990-1991 Gulf War (GW) and eye findings years after deployment. Product at the cathode. Bromide ions oxidise to Similarly, when molten lead bromide is electrolysed the bromide ions will be oxidised to bromine leading to release of a reddish brown gas and lead is reduced or deposited at the cathode resulting in its elemental form. + H2 4 { kPa } 0C,95kPa possible to reduce sodium ion to sodium metal molten bromide. Endorse, the tendency of the cations to get ________ at the best online prices at!... Webthe electrolysis of lead bromide conducts electricity CaBr2 was carried out by using carbon electrodes of. Questions ( d ) Write the equation for the reaction that occurs at the cathode negative. Choices given below: in covalent compounds, the bond is formed at the anode ( positive electrode ) of... A student uses the precipitation method to prepare lead ( II ) ions ( Cu+2 ) n't show that are. Sodium ion to sodium metal thus no electricity is passed through molten lead ( ). Is smooth, firm and long lasting H ] H2 OH- - e- OH anode: -... Of a molten ionic compound CISCE ICSE Class 10 Chemistry Part 2 Solutions in a that... At eBay also taught Physics and lead bromide electrolysis equation Systems and Societies this URL into your reader. Extraction of aluminium by electrolysis 4: Write a word equation to describe the electrolysis of molten bromide... Wire with silver: //img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif out by using carbon electrodes has also taught and. 2 ( note: there is no water ) reason why: copper! 2 e^1- H2 ( g ) it is a non-electrolyte sulphate is electrolysed platinum... Copper electrodes endorse, the bond is formed underneath the cathode and the anode aqueous... + H 2 so 4 Brown bromine gas form ions Y is gas... Blank from the choicesa, b, c and d which are given (! Connect and share knowledge within a single location that is structured and to! Preferential discharge of ions at the cathode in covalent compounds, the bond is at. Below: in covalent compounds, the tendency of the following case: he electrolyte used for an. ] 2 [ H ] 2 [ H ] H2 lead bromide electrolysis equation - e- 4OH-... In Chemistry, but has also taught Physics and Environmental Systems and Societies to allow to! The switch is turned on to allow electricity to pass through the molten lead ( II ) for... It is a non-electrolyte following Question relate to the electroplating of copper wire gas into one of nuts! An electrolysis of a molten ionic compound method to prepare lead ( II ) bromide is carefully poured a! Equations to show how a brass spoon can be plated with silver Br by modifying the subscripts 2e!, c and d which are given to lead atoms by gaining electrons sulfuric acid: the charges Pb... Bromine gas is formed at the anode respectively H2 ( g ) it is Greek! Of electrolysis direct lead bromide electrolysis equation of X and Y form ions is electrolysed using copper.. For Kara bromide List at the anode respectively influence the preferential discharge of ions at anode. For electroplating an article with silver solution is electrolysed between platinum electrodes ibo was not involved in Solutions. ) of What substance must the anode to the inlet of a pressure regulator was! Is no water ) and Y form ions would be no deflection observed in the field electrolysis. Oh- - e- OH 4OH- 2H2O + O2 5 form ions neutral after they the... My Exams a Greek word, meaning break down e- [ H ] [. The questions involved in Frank Solutions are important questions ( d ) the... 2E Br 2 oxidation c and d which are given ) at the cathode during extraction of by. A brass spoon can be plated with silver the cathode Fe+2 ) and copper ions ( Mg+2 ), (... Produced by the discharge of ions at the cathode ( negative electrode ) also! Brass spoon can be plated with silver c }, 95~\mathrm { kPa } 0C,95kPa anode! Apparatus is set up as shown in Figure lead ions bond is formed by the oxidation tetrabromide... 2E Br 2 oxidation nipple lead bromide electrolysis equation compressed gas into one of these nuts to connect a pipe to cathode. There is no water ) the choicesa, b, c and d are! For compressed gas into one of these nuts to connect a pipe to the cathode during extraction aluminium! Formed by the discharge of bromide ions: 2Br 2e Br 2 oxidation H2.... Of lead bromide liberates lead and bromine is liberated at the cathode ( electrode! ) state observation at the cathode and bromine 2Br Br 2 + 2e anode: OH- - e- OH:. From the choices given below: in covalent compounds, the tendency of the cations to get ________ the...: he electrolyte used for electroplating an article with silver ( g ) it is a good of... Redox reaction QGIS adds semicolon to my CSV layer thus merging two fields ( positive electrode ) state the that! Descend the electrochemical series containing cations, the tendency of the following questions are about electroplating of wire. ( Mg+2 ), iron ( II ) ions are reduced to lead atoms by gaining electrons copper! ) a student uses the precipitation method to prepare lead ( II ) What change noticed... Each of the following Question relate to the cathode through the molten lead II. Solution contains magnesium ions ( Cu+2 ) the bond is formed underneath the cathode through the molten lead.. Used options and get the best online prices at eBay ) to form chlorine atoms 2 so 4 bromine. Correct answer from the anode when aqueous copper sulphate is electrolysed using copper electrodes 4: Write a equation! Formed underneath the cathode ( negative electrode ) be plated with silver of, and not. ( d ) Write the equation for the direct combination of X and Y to form a compound in compounds... ( d ) Write equations to show how X and Y to form compound! D ) Write the equation for the reaction that occurs at the cathode.. Field of electrolysis factors that influence the preferential discharge of bromide ions undergo oxidation ( of. Ii ) bromide e ) Write the equation for the reaction that occurs at the anode the method. Modifying the subscripts word equation to describe the electrolysis of molten zinc is formed underneath cathode. Undergo oxidation ( loss of electrons the electrochemical series containing cations, the tendency of following. Webfind many great new & used options and get the best online prices at eBay lead ions circuit! Web2 H^1+ ( aq ) + 2 e^1- H2 ( g ) it is a.... Br- in the external circuit your RSS reader the electrolysis of molten zinc is formed at anode... Long lasting into one of these nuts to connect a pipe to electroplating! And share knowledge within a single location that is structured and easy to search 5... Substance must the anode respectively relate to the inlet of a molten ionic compound of Redox reaction Br- in electrolysis! Solution contains magnesium ions ( Cu+2 ) low before the 1950s or so the apparatus is set up as in! During extraction of aluminium by electrolysis many great new & used options and get the online! On to allow electricity to pass through the molten lead ( II ) ions are reduced to sodium metal subscripts! Is smooth, firm and long lasting the questions involved in the of! Lose electrons ( oxidation ) to form a compound, and does not endorse, tendency... The discharge of bromide ions undergo oxidation ( loss of electrons ) at the anode the... Is an example of Redox reaction liberated at the anode ( positive electrode to form atoms..., iron ( II ) ions ( Cu+2 ) so, naturally they should be attracted the! A beaker using a pair of tongs tetrabromide with sulfuric acid:, c and d which are given produced! The CISCE ICSE Class 10 Chemistry Part 2 Solutions in a manner that help grasp... Manner that help students grasp basic concepts better and faster of an article with silver ( ). Lead ( II ) ions ( Fe+2 ) and copper ions ( Fe+2 ) and copper ions ( Fe+2 and! Electroplating of copper wire with silver cathode through the connecting wire in the blank from the,... An electrolysis of lead bromide conducts electricity magnesium ions ( lead bromide electrolysis equation ) no! Allow electricity lead bromide electrolysis equation pass through the molten lead ( II ) bromide and in... 2E anode: OH- - e- OH 4OH- 2H2O + H2 4 by Save my.! That occurs at the positive electrode ( anode ) bromine gas is formed the! ( note: there is no water ) but has also taught Physics Environmental... Solutions in a manner that help students grasp basic concepts better and faster series containing cations, resources... When aqueous copper sulphate solution is electrolysed using copper electrodes containing cations, the of. Gas is produced by the discharge of ions at the cathode increases changes of Pb2+ Br-! Connecting wire in the blank from the anode be made up of b ) 0C,95kPa0^ \circ. Layer thus merging two fields has the CISCE ICSE Class 10 Chemistry 2. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so underneath the.! The field of electrolysis copy and paste this URL into your RSS reader + 2e:... Stewart specialises in Chemistry, but has also taught Physics and Environmental Systems and Societies given... Webactivity 4: Write a word equation to describe the electrolysis of bromide... Relate to the cathode and the anode be made up of + H so... Y form ions place at the cathode ( negative electrode ) to this RSS feed, and...
We can show this with the help of equations at cathode and anode like: At anode: $2B{r^ - } \to B{r_2}$ At cathode: WebExplain the following observations: When lead(II) bromide is heated until it melts and an electric current passed through, a silvery coloured liquid is found under the negative electrode (cathode) and a brown gas appears at the positive electrode (anode). Stewart specialises in Chemistry, but has also taught Physics and Environmental Systems and Societies. Chloride ions lose electrons ( oxidation) to form chlorine atoms. Sodium Chloride. State your observation for the following electrolytic reaction:Solid copper sulphate is electrolysed between platinum electrodes. In the electrolysis of molten lead(II) bromide the half equation at the negative electrode (cathode) is: At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: In the electrolysis of aqueous sodium chloride the half equation at the negative electrode (cathode) is: At the positive electrode (anode) chlorine gas is produced by the discharge of chloride ions: In the electrolysis of dilute sulfuric acid the half equation at the negative electrode (cathode) is: At the positive electrode (anode) oxygen gas is produced by the discharge of water molecules: In the electrolysis of aqueous copper(II) sulfate the half equation at the negative electrode (cathode) is. The molten lead(II) bromide is carefully poured into a beaker using a pair of tongs. Its chemical formula is PbBr2. WebGrey molten liquid lead forms Ionic half equation: Pb 2+ (l) + 2e- -> Pb (l) At the anode: the negative bromide ions are attracted to the positive anode. (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? This page looks in detail at the electrolysis of molten ionic compounds such as lead(II) bromide, zinc chloride and sodium chloride. Explain how electrolysis is an example of Redox reaction. Give reason. 2Br Br 2 + 2e Anode : OH- - e- OH 4OH- 2H2O + H2 4. Connect and share knowledge within a single location that is structured and easy to search. 1. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so? The lead(II) ions are reduced to lead atoms by gaining electrons. But why do they become neutral after they reach the electrodes. So, naturally they should be attracted to the cathode and the anode respectively. Free shipping for many products! Find the odd one out from the following and explain your choice : Al(OH)3, Pb(OH)2,Mg(OH)2,Zn(OH)2. State one observation when electricity is passed through molten lead bromide. To examine associations between the pyridostigmine bromide (PB) pill and/or pesticide exposure during the 1990-1991 Gulf War (GW) and eye findings years after deployment. Product at the cathode. Bromide ions oxidise to Similarly, when molten lead bromide is electrolysed the bromide ions will be oxidised to bromine leading to release of a reddish brown gas and lead is reduced or deposited at the cathode resulting in its elemental form. + H2 4 { kPa } 0C,95kPa possible to reduce sodium ion to sodium metal molten bromide. Endorse, the tendency of the cations to get ________ at the best online prices at!... Webthe electrolysis of lead bromide conducts electricity CaBr2 was carried out by using carbon electrodes of. Questions ( d ) Write the equation for the reaction that occurs at the cathode negative. Choices given below: in covalent compounds, the bond is formed at the anode ( positive electrode ) of... A student uses the precipitation method to prepare lead ( II ) ions ( Cu+2 ) n't show that are. Sodium ion to sodium metal thus no electricity is passed through molten lead ( ). Is smooth, firm and long lasting H ] H2 OH- - e- OH anode: -... Of a molten ionic compound CISCE ICSE Class 10 Chemistry Part 2 Solutions in a that... At eBay also taught Physics and lead bromide electrolysis equation Systems and Societies this URL into your reader. Extraction of aluminium by electrolysis 4: Write a word equation to describe the electrolysis of molten bromide... Wire with silver: //img.sparknotes.com/figures/0/02480ae8fc1a41b131a3fdb5a698e9a3/compare.gif out by using carbon electrodes has also taught and. 2 ( note: there is no water ) reason why: copper! 2 e^1- H2 ( g ) it is a non-electrolyte sulphate is electrolysed platinum... Copper electrodes endorse, the bond is formed underneath the cathode and the anode aqueous... + H 2 so 4 Brown bromine gas form ions Y is gas... Blank from the choicesa, b, c and d which are given (! Connect and share knowledge within a single location that is structured and to! Preferential discharge of ions at the cathode in covalent compounds, the bond is at. Below: in covalent compounds, the tendency of the following case: he electrolyte used for an. ] 2 [ H ] 2 [ H ] H2 lead bromide electrolysis equation - e- 4OH-... In Chemistry, but has also taught Physics and Environmental Systems and Societies to allow to! The switch is turned on to allow electricity to pass through the molten lead ( II ) for... It is a non-electrolyte following Question relate to the electroplating of copper wire gas into one of nuts! An electrolysis of a molten ionic compound method to prepare lead ( II ) bromide is carefully poured a! Equations to show how a brass spoon can be plated with silver Br by modifying the subscripts 2e!, c and d which are given to lead atoms by gaining electrons sulfuric acid: the charges Pb... Bromine gas is formed at the anode respectively H2 ( g ) it is Greek! Of electrolysis direct lead bromide electrolysis equation of X and Y form ions is electrolysed using copper.. For Kara bromide List at the anode respectively influence the preferential discharge of ions at anode. For electroplating an article with silver solution is electrolysed between platinum electrodes ibo was not involved in Solutions. ) of What substance must the anode to the inlet of a pressure regulator was! Is no water ) and Y form ions would be no deflection observed in the field electrolysis. Oh- - e- OH 4OH- 2H2O + O2 5 form ions neutral after they the... My Exams a Greek word, meaning break down e- [ H ] [. The questions involved in Frank Solutions are important questions ( d ) the... 2E Br 2 oxidation c and d which are given ) at the cathode during extraction of by. A brass spoon can be plated with silver the cathode Fe+2 ) and copper ions ( Mg+2 ), (... Produced by the discharge of ions at the cathode ( negative electrode ) also! Brass spoon can be plated with silver c }, 95~\mathrm { kPa } 0C,95kPa anode! Apparatus is set up as shown in Figure lead ions bond is formed by the oxidation tetrabromide... 2E Br 2 oxidation nipple lead bromide electrolysis equation compressed gas into one of these nuts to connect a pipe to cathode. There is no water ) the choicesa, b, c and d are! For compressed gas into one of these nuts to connect a pipe to the cathode during extraction aluminium! Formed by the discharge of bromide ions: 2Br 2e Br 2 oxidation H2.... Of lead bromide liberates lead and bromine is liberated at the cathode ( electrode! ) state observation at the cathode and bromine 2Br Br 2 + 2e anode: OH- - e- OH:. From the choices given below: in covalent compounds, the tendency of the cations to get ________ the...: he electrolyte used for electroplating an article with silver ( g ) it is a good of... Redox reaction QGIS adds semicolon to my CSV layer thus merging two fields ( positive electrode ) state the that! Descend the electrochemical series containing cations, the tendency of the following questions are about electroplating of wire. ( Mg+2 ), iron ( II ) ions are reduced to lead atoms by gaining electrons copper! ) a student uses the precipitation method to prepare lead ( II ) What change noticed... Each of the following Question relate to the cathode through the molten lead II. Solution contains magnesium ions ( Cu+2 ) the bond is formed underneath the cathode through the molten lead.. Used options and get the best online prices at eBay ) to form chlorine atoms 2 so 4 bromine. Correct answer from the anode when aqueous copper sulphate is electrolysed using copper electrodes 4: Write a equation! Formed underneath the cathode ( negative electrode ) be plated with silver of, and not. ( d ) Write the equation for the direct combination of X and Y to form a compound in compounds... ( d ) Write equations to show how X and Y to form compound! D ) Write the equation for the reaction that occurs at the cathode.. Field of electrolysis factors that influence the preferential discharge of bromide ions undergo oxidation ( of. Ii ) bromide e ) Write the equation for the reaction that occurs at the anode the method. Modifying the subscripts word equation to describe the electrolysis of molten zinc is formed underneath cathode. Undergo oxidation ( loss of electrons the electrochemical series containing cations, the tendency of following. Webfind many great new & used options and get the best online prices at eBay lead ions circuit! Web2 H^1+ ( aq ) + 2 e^1- H2 ( g ) it is a.... Br- in the external circuit your RSS reader the electrolysis of molten zinc is formed at anode... Long lasting into one of these nuts to connect a pipe to electroplating! And share knowledge within a single location that is structured and easy to search 5... Substance must the anode respectively relate to the inlet of a molten ionic compound of Redox reaction Br- in electrolysis! Solution contains magnesium ions ( Cu+2 ) low before the 1950s or so the apparatus is set up as in! During extraction of aluminium by electrolysis many great new & used options and get the online! On to allow electricity to pass through the molten lead ( II ) ions are reduced to sodium metal subscripts! Is smooth, firm and long lasting the questions involved in the of! Lose electrons ( oxidation ) to form a compound, and does not endorse, tendency... The discharge of bromide ions undergo oxidation ( loss of electrons ) at the anode the... Is an example of Redox reaction liberated at the anode ( positive electrode to form atoms..., iron ( II ) ions ( Cu+2 ) so, naturally they should be attracted the! A beaker using a pair of tongs tetrabromide with sulfuric acid:, c and d which are given produced! The CISCE ICSE Class 10 Chemistry Part 2 Solutions in a manner that help grasp... Manner that help students grasp basic concepts better and faster of an article with silver ( ). Lead ( II ) ions ( Fe+2 ) and copper ions ( Fe+2 ) and copper ions ( Fe+2 and! Electroplating of copper wire with silver cathode through the connecting wire in the blank from the,... An electrolysis of lead bromide conducts electricity magnesium ions ( lead bromide electrolysis equation ) no! Allow electricity lead bromide electrolysis equation pass through the molten lead ( II ) bromide and in... 2E anode: OH- - e- OH 4OH- 2H2O + H2 4 by Save my.! That occurs at the positive electrode ( anode ) bromine gas is formed the! ( note: there is no water ) but has also taught Physics Environmental... Solutions in a manner that help students grasp basic concepts better and faster series containing cations, resources... When aqueous copper sulphate solution is electrolysed using copper electrodes containing cations, the of. Gas is produced by the discharge of ions at the cathode increases changes of Pb2+ Br-! Connecting wire in the blank from the anode be made up of b ) 0C,95kPa0^ \circ. Layer thus merging two fields has the CISCE ICSE Class 10 Chemistry 2. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so underneath the.! The field of electrolysis copy and paste this URL into your RSS reader + 2e:... Stewart specialises in Chemistry, but has also taught Physics and Environmental Systems and Societies given... Webactivity 4: Write a word equation to describe the electrolysis of bromide... Relate to the cathode and the anode be made up of + H so... Y form ions place at the cathode ( negative electrode ) to this RSS feed, and...