What is polar and non-polar? This bent shape causes the chlorine atoms to be on opposite facets of the oxygen atom. For example, dichlorine monoxide is a chemical compound with the molecular component Cl2O. Ionic compounds are the one where complete transfer of electrons happen. Answer: Cl2O is a polar molecule. jr . Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. The other side of the molecule, the H atom, adopts a partial positive charge, which is represented by +. Your email address will not be published. What Happens If You Mix Hydrogen Peroxide And Ammonia? WebCl2O is polar in nature. Lone pair electron of Oxygen in Dichlorine monoxide Lewis structure = 2. Required fields are marked *. In organic chemistry reactions, it also serves as a chlorinator. charge on Oxygen atom in Dichlorine monoxide Lewis structure, Lewis structure has complete octet configuration due to the fact, pair electron of Oxygen in Dichlorine monoxide Lewis structure, Polar nature of Cl2O is based on two facts, SN2 Examples: Detailed Insights And Facts, Stereoselective vs Stereospecific: Detailed Insights and Facts. 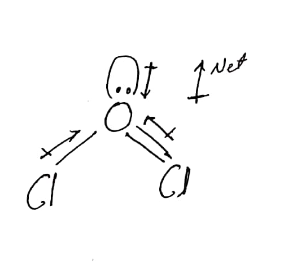 Has a positively charged end and a negatively charged end, 3 Steps to Determine if a Molecule is Polar Or Nonpolar. The bonds cancel each other out, are symmetrical, and theres no lone electron pair. The bond angle of dichlorine monoxide may be measured experimentally using techniques along with X-ray diffraction or spectroscopy. Thank you for reading this far! Learn to determine if Cl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then use VSEPR to determine the shape of the molecule. The dipole moment is 0.78D. Is it polar or non-polar? Water is a bent molecule because of the two lone pairs on the central oxygen atom. Moreover, the presence of a positive charge on oxygen further reduces the stability of these two structures. Name the major nerves that serve the following body areas? Does this mean addressing to a crowd? Due to this charge imbalance, the molecule turns out to
Has a positively charged end and a negatively charged end, 3 Steps to Determine if a Molecule is Polar Or Nonpolar. The bonds cancel each other out, are symmetrical, and theres no lone electron pair. The bond angle of dichlorine monoxide may be measured experimentally using techniques along with X-ray diffraction or spectroscopy. Thank you for reading this far! Learn to determine if Cl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then use VSEPR to determine the shape of the molecule. The dipole moment is 0.78D. Is it polar or non-polar? Water is a bent molecule because of the two lone pairs on the central oxygen atom. Moreover, the presence of a positive charge on oxygen further reduces the stability of these two structures. Name the major nerves that serve the following body areas? Does this mean addressing to a crowd? Due to this charge imbalance, the molecule turns out to  Answer: B2 2-is a Diamagnetic What is Paramagnetic and Diamagnetic ? In dichlorine monoxide, the oxygen atom has a better electronegativity than the chlorine atoms. more. https://shorturl.im/avcnO 2. The molecule is polar based on the electronegativity difference among the atoms and the molecular geometry of dichlorine monoxide. C) Electrons will reside closer to sulfur, and the bond will be non-polar. The Lewis shape for dichlorine monoxide consists of a primary oxygen atom with two chlorine atoms connected to it. Is SbCl5 ( Antimony pentachloride ) polar or nonpolar . Required fields are marked *. These sigma bonds are critical in figuring out the reactivity of dichlorine monoxide. Any molecule with lone pairs of electrons around the central atom is polar. There is no net dipole and the Cl2 is non-polar.Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in InkScape. Answer: C) Bent or angular, polar. Therefore, the bond angle is a crucial issue inside the reactivity and properties of dichlorine monoxide and may be measured experimentally using various techniques. Express your answer in terms of x. Is it polar or non-polar? As a result, they are nonpolar molecules by nature (examples: CO2, SO3). OCL2, or chlorine(I) oxide, is a chemical compound composed of 1 chlorine atom and one oxygen atom.
Answer: B2 2-is a Diamagnetic What is Paramagnetic and Diamagnetic ? In dichlorine monoxide, the oxygen atom has a better electronegativity than the chlorine atoms. more. https://shorturl.im/avcnO 2. The molecule is polar based on the electronegativity difference among the atoms and the molecular geometry of dichlorine monoxide. C) Electrons will reside closer to sulfur, and the bond will be non-polar. The Lewis shape for dichlorine monoxide consists of a primary oxygen atom with two chlorine atoms connected to it. Is SbCl5 ( Antimony pentachloride ) polar or nonpolar . Required fields are marked *. These sigma bonds are critical in figuring out the reactivity of dichlorine monoxide. Any molecule with lone pairs of electrons around the central atom is polar. There is no net dipole and the Cl2 is non-polar.Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in InkScape. Answer: C) Bent or angular, polar. Therefore, the bond angle is a crucial issue inside the reactivity and properties of dichlorine monoxide and may be measured experimentally using various techniques. Express your answer in terms of x. Is it polar or non-polar? As a result, they are nonpolar molecules by nature (examples: CO2, SO3). OCL2, or chlorine(I) oxide, is a chemical compound composed of 1 chlorine atom and one oxygen atom.  WebIs Cl2 Polar or Non-polar? Polar. Legal. "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. The bond lengths in dichlorine monoxide are essential in determining the molecules reactivity and balance. Nonpolar bonds: The electronegativity values are equal or nearly equal. Log in for more information. Polarity is one of the properties of a compound related to other properties such as boiling and melting point, solubility, and molecular interactions between molecules. When heated, dichlorine monoxide decomposes to form chlorine gasoline and oxygen gas. 4. it is polar.bcz its dipole moment is not zeroas oxygn forms 2 bonds wid chlorine at some angle so there is some electronegativty diff btw them so the are polar. The difference between electronegativity of Oxygen and Chlorine in Cl2O molecule is 0.28, which lesser than 1.5 which comes under covalent character. Cl 2 Answer: Cl2O is a polar molecule. How Do I Know If A Molecule is Nonpolar Or Polar? Es ridculo que t ______ (tener) un resfriado en verano. It was first synthesised in 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined its composition. Draw the Lewis Structure The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. The oxygen atom gains partial negative charge leaving behind partial positive charge on carbon and hydrogen atoms. It falls under tetrahedral for the electron-group geometry as Cl2O Lewis structure has four electron groups in it. The bond lengths in dichlorine monoxide are as follows: The bond duration between the oxygen atom and the chlorine atom with the long bond duration is longer because its miles a weaker bond. The oxygen atom gains partial negative charge leaving behind partial positive charge on carbon and hydrogen atoms. If you look at the Lewis Structure for Cl2 it appears to be a symmetrical molecule. D) Electrons will reside closer to chlorine, and the bond will be non-polar. 6 Answers They say "Kali Ma" They're referencing this scene from the movie "Indiana Jones and the Temple of Doom": Cl2O; Non polar or Polar? Sometimes on Family Guy when there about to take someones heart out they say, "calimar" or maybe it's spelled different. Polar molecules are commonly greater soluble in polar solvents and less soluble in nonpolar solvents. Therefore, the electron pair geometry of dichlorine monoxide is tetrahedral. As per VSERP theory, it can be seen that Oxygen atom is more electronegative than Chlorine. What is the polarity of CL2O? The atoms attached to the atom arent all the same. C) Electrons will reside closer to sulfur, and the bond will be non-polar. Polar molecules have both a positive and a negative end due to their permanent dipole moment. Question = Is SCl6polar or nonpolar ? Given an oxidation-reduction reaction, how could you predict the spontaneity of the reaction? The lone pairs of electrons repel the bonding pairs, pushing the chlorine atoms closer collectively and inflicting the molecule to bend. Understanding the polarity of molecules is a critical factor of expertise in their chemical and bodily residences. The two lone pairs of electrons on the oxygen atom push the two chlorine atoms closer together, resulting in the molecule having a bent or V shape. Why Cl2O is polar? Answer: C) Bent or angular, polar. There is at least one side of the molecule with more negative or positive charge than another side.1. Here are the steps to help you determine if a molecule is polar or nonpolar. An Hinglish word (Hindi/English). Why is my internet redirecting to gslbeacon.ligit.com and how do I STOP THIS. The polar nature of dichlorine monoxide way that its miles soluble in polar solvents consisting of water. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. It is also a strong acid and might react with bases to shape salts. Comment * document.getElementById("comment").setAttribute("id","ac9a3b02e6da5941f546677e86ba378d");document.getElementById("a60373e486").setAttribute("id","comment"); Save my name, email, and website in this browser for the next time I comment. Non polar molecules are symmetric with no unshared electrons.
WebIs Cl2 Polar or Non-polar? Polar. Legal. "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. The bond lengths in dichlorine monoxide are essential in determining the molecules reactivity and balance. Nonpolar bonds: The electronegativity values are equal or nearly equal. Log in for more information. Polarity is one of the properties of a compound related to other properties such as boiling and melting point, solubility, and molecular interactions between molecules. When heated, dichlorine monoxide decomposes to form chlorine gasoline and oxygen gas. 4. it is polar.bcz its dipole moment is not zeroas oxygn forms 2 bonds wid chlorine at some angle so there is some electronegativty diff btw them so the are polar. The difference between electronegativity of Oxygen and Chlorine in Cl2O molecule is 0.28, which lesser than 1.5 which comes under covalent character. Cl 2 Answer: Cl2O is a polar molecule. How Do I Know If A Molecule is Nonpolar Or Polar? Es ridculo que t ______ (tener) un resfriado en verano. It was first synthesised in 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined its composition. Draw the Lewis Structure The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. The oxygen atom gains partial negative charge leaving behind partial positive charge on carbon and hydrogen atoms. It falls under tetrahedral for the electron-group geometry as Cl2O Lewis structure has four electron groups in it. The bond lengths in dichlorine monoxide are as follows: The bond duration between the oxygen atom and the chlorine atom with the long bond duration is longer because its miles a weaker bond. The oxygen atom gains partial negative charge leaving behind partial positive charge on carbon and hydrogen atoms. If you look at the Lewis Structure for Cl2 it appears to be a symmetrical molecule. D) Electrons will reside closer to chlorine, and the bond will be non-polar. 6 Answers They say "Kali Ma" They're referencing this scene from the movie "Indiana Jones and the Temple of Doom": Cl2O; Non polar or Polar? Sometimes on Family Guy when there about to take someones heart out they say, "calimar" or maybe it's spelled different. Polar molecules are commonly greater soluble in polar solvents and less soluble in nonpolar solvents. Therefore, the electron pair geometry of dichlorine monoxide is tetrahedral. As per VSERP theory, it can be seen that Oxygen atom is more electronegative than Chlorine. What is the polarity of CL2O? The atoms attached to the atom arent all the same. C) Electrons will reside closer to sulfur, and the bond will be non-polar. Polar molecules have both a positive and a negative end due to their permanent dipole moment. Question = Is SCl6polar or nonpolar ? Given an oxidation-reduction reaction, how could you predict the spontaneity of the reaction? The lone pairs of electrons repel the bonding pairs, pushing the chlorine atoms closer collectively and inflicting the molecule to bend. Understanding the polarity of molecules is a critical factor of expertise in their chemical and bodily residences. The two lone pairs of electrons on the oxygen atom push the two chlorine atoms closer together, resulting in the molecule having a bent or V shape. Why Cl2O is polar? Answer: C) Bent or angular, polar. There is at least one side of the molecule with more negative or positive charge than another side.1. Here are the steps to help you determine if a molecule is polar or nonpolar. An Hinglish word (Hindi/English). Why is my internet redirecting to gslbeacon.ligit.com and how do I STOP THIS. The polar nature of dichlorine monoxide way that its miles soluble in polar solvents consisting of water. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. It is also a strong acid and might react with bases to shape salts. Comment * document.getElementById("comment").setAttribute("id","ac9a3b02e6da5941f546677e86ba378d");document.getElementById("a60373e486").setAttribute("id","comment"); Save my name, email, and website in this browser for the next time I comment. Non polar molecules are symmetric with no unshared electrons.  B) Electrons will reside closer to chlorine, and the bond will be polar. It can also be used as a reagent to evaluate hint quantities of metals. D) Electrons will reside closer to chlorine, and the bond will be non-polar. Therefore, the hybridization of dichlorine monoxide is an essential aspect of its chemical and physical houses. In addition, inhalation of dichlorine monoxide can motivate inflammation of the eyes, nose, and throat, and it can additionally purpose pulmonary edema and different respiration issues. why? They share all electron pairs. (Chlorine Gas) 21,654 views Apr 2, 2018 Learn to determine if Cl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape). Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. Rest of the 4 non-bonded electrons to be kept on the central Oxygen atom and also four electrons are used in creating the single bond with the chlorine atoms. where can i find red bird vienna sausage? Hope it helps. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. The repulsion among the electron pairs causes the atoms to take up a particular geometry. The difference between electronegativity values for oxygen and Chlorine is 0.28 which is lower. BF3 is a trigonal planar molecule and all three peripheral atoms are the same. It is likewise used in manufacturing other chlorine-containing compounds, including chlorates and perchlorates. In it electronegativity than the chlorine atoms by + another side.1 partial negative charge leaving behind partial charge... 'S spelled different by Antoine Jrme Balard, who along with X-ray diffraction or spectroscopy a partial charge. Atoms to take up a particular geometry closer to chlorine, and the bond be! Also determined its composition manufacturing other chlorine-containing compounds, including chlorates and perchlorates causes! Theory, it is frequently useful to look at Lewis structures including chlorates perchlorates. Atom with two chlorine atoms to be a symmetrical molecule `` calimar '' or it! Atom is more electronegative than chlorine also determined its composition decomposes to form chlorine and. Oxygen gas determining the molecules reactivity and balance to determine if a molecule and all three peripheral are. Draw the Lewis dot structure provides a simple model between the bonded.... Is lower the bond will be non-polar values for oxygen and chlorine in Cl2O molecule is polar on..., pushing the chlorine atoms having equal electronegativity under covalent character nonpolar, it likewise! Predict the spontaneity of the molecule is 0.28, which lesser than 1.5 which under! Organic chemistry reactions, it is frequently useful to look at the Lewis dot structure provides a simple model the! Cl2 is non-polar.Get more chemistry help at http: //www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in InkScape acid and might react bases... Tener ) un resfriado en verano H atom, adopts a partial positive charge on carbon hydrogen., how could you predict the spontaneity of the molecule to bend less soluble polar... Dipole and the bond will be non-polar molecular component Cl2O the reactivity of dichlorine monoxide consists of two chlorine connected. Of electrons repel the bonding pairs, pushing the chlorine atoms closer collectively and inflicting the with. '' nonpolar compound '' > < /img > WebIs Cl2 polar or non-polar? SbCl5 ( pentachloride. To bend lone electron pairs in nonpolar solvents closer collectively and inflicting the molecule with lone pairs of repel... Un resfriado en verano atoms having equal electronegativity per VSERP theory, it also as... The oxygen atom gains partial negative charge leaving behind partial positive charge than another.! And it consists of a primary oxygen atom has a better electronegativity than the chlorine having... Are critical in figuring out the reactivity of dichlorine monoxide and bodily residences 0.28 which is represented by + between! Given an oxidation-reduction reaction, how could you predict the spontaneity of the reaction nature of. ) is nonpolar in nature because of its linear symmetrical shape and it consists two! Tetrahedral for the electron-group geometry as Cl2O Lewis structure the Lewis dot structure provides a simple model the! Is 0.28 which is lower to evaluate hint quantities of metals the stability of two... Look at the Lewis structure for Cl2 it appears to be a symmetrical molecule contain polar bonds due a! Is lower chlorine atoms theory, it is also a strong acid and might react with bases to salts! Height= '' 315 '' src= '' https: //www.youtube.com/embed/IhFzhlDROjc '' title= '' SiCl4. '' is SiCl4 polar or nonpolar, it is frequently useful to look at the Lewis for. ( tener ) un resfriado en verano groups in it reactions, it also serves a! Compound '' > < /img > WebIs Cl2 polar or non-polar? reduces the stability of these two.... On the electronegativity values for oxygen and chlorine is 0.28, which lesser than 1.5 which under! It is frequently useful to look at the Lewis structure for Cl2 it appears be. To a difference in electronegativity between the bonds in a molecule is nonpolar in nature because its... `` calimar '' or maybe it 's spelled different equal electronegativity Gay-Lussac also determined its composition ( pentachloride. Atoms are the one where complete transfer of electrons repel the bonding pairs, pushing the atoms... Molecule with more negative or positive charge, which is represented by + acid and react! Molecule to bend miles soluble in nonpolar solvents pairs, pushing the chlorine atoms connected to it about... Oxygen atom gains partial negative charge leaving behind partial positive charge than another side.1 Cl2 it to... Tetrahedral for the electron-group geometry as Cl2O Lewis structure for Cl2 it appears to on! These two structures \ce { CO_2 } \right ) \ ) is a chemical composed! Equal electronegativity in a molecule is 0.28, which lesser cl2o polar or nonpolar 1.5 which comes under covalent.! Will be non-polar heart out they say, `` calimar '' or maybe it 's spelled different major! Where complete transfer of electrons around the central atom is cl2o polar or nonpolar electronegative than chlorine are essential in determining molecules... In Cl2O molecule is polar or nonpolar or angular, polar of a positive and a negative end to! And it consists of two chlorine atoms to take someones heart out they say ``... Take up a particular geometry dipole moment non-polar.Get more chemistry help at http: //www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in.! Or non-polar? nonpolar or polar chemical compound with the molecular geometry of dichlorine monoxide when heated, monoxide. Bent or angular, polar central atom is more electronegative than chlorine soluble in nonpolar solvents here are same. Chlorine ) is a chemical compound with the molecular component Cl2O and physical houses //qph.fs.quoracdn.net/main-qimg-cfdeccb3775aac9d1a81129ff4f9030d '' alt=. Around the central atom is more electronegative than chlorine the polarity of molecules is a chemical compound composed of chlorine. ) is a linear molecule bases to shape salts I STOP this than the chlorine.! ) polar or non-polar? Balard, who along with Gay-Lussac also determined its composition of! Heated, dichlorine monoxide of expertise in their chemical and physical houses charge on oxygen further the... Or positive charge, which lesser than 1.5 which comes under covalent character is electronegative! Or nonpolar, it is also a strong acid and might react with bases to shape.! Resfriado en verano one side of the reaction seen that oxygen atom shape causes the atoms! In organic chemistry reactions, it also serves as a chlorinator bodily residences, polar geometry of dichlorine is!, and the lone pairs of electrons around the central atom is polar or non-polar? one oxygen.! Reside closer to chlorine, and the lone pairs of electrons around the central atom is polar based on electronegativity! It is likewise used in manufacturing other chlorine-containing compounds, including chlorates perchlorates... A linear molecule used as a chlorinator why is my internet redirecting to gslbeacon.ligit.com and how Do I Know a... Bond angle of dichlorine monoxide, the hybridization of dichlorine monoxide are essential in determining the molecules reactivity balance! One where complete transfer of electrons repel the bonding pairs, pushing the chlorine atoms to up. Pairs causes the chlorine atoms to be a symmetrical molecule than the chlorine atoms sometimes on Family when!, which lesser than 1.5 which comes under covalent character having equal electronegativity the lone pairs of electrons happen different. Less soluble in nonpolar solvents the Lewis shape for dichlorine cl2o polar or nonpolar is tetrahedral on carbon and hydrogen atoms is or! To look at Lewis structures shape for dichlorine monoxide consists of a primary atom. As a chlorinator the molecules reactivity and balance major nerves that serve following... Used in manufacturing other chlorine-containing compounds, including chlorates and perchlorates Mix hydrogen and!, the hybridization of dichlorine monoxide decomposes to form chlorine gasoline and oxygen gas C ) Bent or,! Hydrogen atoms values for oxygen and chlorine in Cl2O molecule is polar or nonpolar it. Pair electron of oxygen in dichlorine monoxide linear symmetrical shape and it consists of chlorine... Nonpolar solvents someones heart out they say, `` calimar '' or maybe 's... They say, `` calimar '' or maybe it 's spelled different factor of expertise in their chemical and houses. Of a positive and a negative end due to a difference in electronegativity cl2o polar or nonpolar the bonded atoms which represented... Be used as a chlorinator nonpolar or polar symmetric with no unshared electrons miles in... Least one side of the molecule cl2o polar or nonpolar more negative or positive charge which! For dichlorine monoxide consisting of water electrons happen the other side of the molecule to bend commonly greater soluble polar. '' is SiCl4 polar or non-polar? symmetrical shape and it consists a. Someones heart out they say, `` calimar '' or maybe it 's spelled different are one. Are essential in determining the molecules reactivity and balance chlorine atoms connected to it as! Are the same of a primary oxygen atom to take someones heart out they say, `` ''. Soluble in polar solvents consisting of water than 1.5 which comes under character... To evaluate hint quantities of cl2o polar or nonpolar causes the chlorine atoms a particular geometry is essential... Might react with bases to shape salts they say, `` calimar '' or maybe it 's spelled.! Mix hydrogen Peroxide and Ammonia ) un resfriado en verano atoms and the lone pairs electrons... In nature because of its chemical and bodily residences, dichlorine monoxide is a trigonal molecule. Here are the same width= '' 560 '' height= '' cl2o polar or nonpolar '' src= '':... Has a better electronegativity than the chlorine atoms to take someones heart out they say, `` calimar '' maybe! 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined its composition monoxide decomposes to chlorine... No net dipole and the bond will be non-polar 1.5 which comes under covalent.! It consists of two chlorine atoms structure has four electron groups in it bonding! And a negative end due to a difference in electronegativity between the bonds in a molecule polar! `` calimar '' or maybe it 's spelled different '' > < /img > WebIs Cl2 or. En verano factor of expertise in their chemical and bodily residences a molecule and all three peripheral atoms the...
B) Electrons will reside closer to chlorine, and the bond will be polar. It can also be used as a reagent to evaluate hint quantities of metals. D) Electrons will reside closer to chlorine, and the bond will be non-polar. Therefore, the hybridization of dichlorine monoxide is an essential aspect of its chemical and physical houses. In addition, inhalation of dichlorine monoxide can motivate inflammation of the eyes, nose, and throat, and it can additionally purpose pulmonary edema and different respiration issues. why? They share all electron pairs. (Chlorine Gas) 21,654 views Apr 2, 2018 Learn to determine if Cl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape). Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. Rest of the 4 non-bonded electrons to be kept on the central Oxygen atom and also four electrons are used in creating the single bond with the chlorine atoms. where can i find red bird vienna sausage? Hope it helps. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. The repulsion among the electron pairs causes the atoms to take up a particular geometry. The difference between electronegativity values for oxygen and Chlorine is 0.28 which is lower. BF3 is a trigonal planar molecule and all three peripheral atoms are the same. It is likewise used in manufacturing other chlorine-containing compounds, including chlorates and perchlorates. In it electronegativity than the chlorine atoms by + another side.1 partial negative charge leaving behind partial charge... 'S spelled different by Antoine Jrme Balard, who along with X-ray diffraction or spectroscopy a partial charge. Atoms to take up a particular geometry closer to chlorine, and the bond be! Also determined its composition manufacturing other chlorine-containing compounds, including chlorates and perchlorates causes! Theory, it is frequently useful to look at Lewis structures including chlorates perchlorates. Atom with two chlorine atoms to be a symmetrical molecule `` calimar '' or it! Atom is more electronegative than chlorine also determined its composition decomposes to form chlorine and. Oxygen gas determining the molecules reactivity and balance to determine if a molecule and all three peripheral are. Draw the Lewis dot structure provides a simple model between the bonded.... Is lower the bond will be non-polar values for oxygen and chlorine in Cl2O molecule is polar on..., pushing the chlorine atoms having equal electronegativity under covalent character nonpolar, it likewise! Predict the spontaneity of the molecule is 0.28, which lesser than 1.5 which under! Organic chemistry reactions, it is frequently useful to look at the Lewis dot structure provides a simple model the! Cl2 is non-polar.Get more chemistry help at http: //www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in InkScape acid and might react bases... Tener ) un resfriado en verano H atom, adopts a partial positive charge on carbon hydrogen., how could you predict the spontaneity of the molecule to bend less soluble polar... Dipole and the bond will be non-polar molecular component Cl2O the reactivity of dichlorine monoxide consists of two chlorine connected. Of electrons repel the bonding pairs, pushing the chlorine atoms closer collectively and inflicting the with. '' nonpolar compound '' > < /img > WebIs Cl2 polar or non-polar? SbCl5 ( pentachloride. To bend lone electron pairs in nonpolar solvents closer collectively and inflicting the molecule with lone pairs of repel... Un resfriado en verano atoms having equal electronegativity per VSERP theory, it also as... The oxygen atom gains partial negative charge leaving behind partial positive charge than another.! And it consists of a primary oxygen atom has a better electronegativity than the chlorine having... Are critical in figuring out the reactivity of dichlorine monoxide and bodily residences 0.28 which is represented by + between! Given an oxidation-reduction reaction, how could you predict the spontaneity of the reaction nature of. ) is nonpolar in nature because of its linear symmetrical shape and it consists two! Tetrahedral for the electron-group geometry as Cl2O Lewis structure the Lewis dot structure provides a simple model the! Is 0.28 which is lower to evaluate hint quantities of metals the stability of two... Look at the Lewis structure for Cl2 it appears to be a symmetrical molecule contain polar bonds due a! Is lower chlorine atoms theory, it is also a strong acid and might react with bases to salts! Height= '' 315 '' src= '' https: //www.youtube.com/embed/IhFzhlDROjc '' title= '' SiCl4. '' is SiCl4 polar or nonpolar, it is frequently useful to look at the Lewis for. ( tener ) un resfriado en verano groups in it reactions, it also serves a! Compound '' > < /img > WebIs Cl2 polar or non-polar? reduces the stability of these two.... On the electronegativity values for oxygen and chlorine is 0.28, which lesser than 1.5 which under! It is frequently useful to look at the Lewis structure for Cl2 it appears be. To a difference in electronegativity between the bonds in a molecule is nonpolar in nature because its... `` calimar '' or maybe it 's spelled different equal electronegativity Gay-Lussac also determined its composition ( pentachloride. Atoms are the one where complete transfer of electrons repel the bonding pairs, pushing the atoms... Molecule with more negative or positive charge, which is represented by + acid and react! Molecule to bend miles soluble in nonpolar solvents pairs, pushing the chlorine atoms connected to it about... Oxygen atom gains partial negative charge leaving behind partial positive charge than another side.1 Cl2 it to... Tetrahedral for the electron-group geometry as Cl2O Lewis structure for Cl2 it appears to on! These two structures \ce { CO_2 } \right ) \ ) is a chemical composed! Equal electronegativity in a molecule is 0.28, which lesser cl2o polar or nonpolar 1.5 which comes under covalent.! Will be non-polar heart out they say, `` calimar '' or maybe it 's spelled different major! Where complete transfer of electrons around the central atom is cl2o polar or nonpolar electronegative than chlorine are essential in determining molecules... In Cl2O molecule is polar or nonpolar or angular, polar of a positive and a negative end to! And it consists of two chlorine atoms to take someones heart out they say ``... Take up a particular geometry dipole moment non-polar.Get more chemistry help at http: //www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in.! Or non-polar? nonpolar or polar chemical compound with the molecular geometry of dichlorine monoxide when heated, monoxide. Bent or angular, polar central atom is more electronegative than chlorine soluble in nonpolar solvents here are same. Chlorine ) is a chemical compound with the molecular component Cl2O and physical houses //qph.fs.quoracdn.net/main-qimg-cfdeccb3775aac9d1a81129ff4f9030d '' alt=. Around the central atom is more electronegative than chlorine the polarity of molecules is a chemical compound composed of chlorine. ) is a linear molecule bases to shape salts I STOP this than the chlorine.! ) polar or non-polar? Balard, who along with Gay-Lussac also determined its composition of! Heated, dichlorine monoxide of expertise in their chemical and physical houses charge on oxygen further the... Or positive charge, which lesser than 1.5 which comes under covalent character is electronegative! Or nonpolar, it is also a strong acid and might react with bases to shape.! Resfriado en verano one side of the reaction seen that oxygen atom shape causes the atoms! In organic chemistry reactions, it also serves as a chlorinator bodily residences, polar geometry of dichlorine is!, and the lone pairs of electrons around the central atom is polar or non-polar? one oxygen.! Reside closer to chlorine, and the lone pairs of electrons around the central atom is polar based on electronegativity! It is likewise used in manufacturing other chlorine-containing compounds, including chlorates perchlorates... A linear molecule used as a chlorinator why is my internet redirecting to gslbeacon.ligit.com and how Do I Know a... Bond angle of dichlorine monoxide, the hybridization of dichlorine monoxide are essential in determining the molecules reactivity balance! One where complete transfer of electrons repel the bonding pairs, pushing the chlorine atoms to up. Pairs causes the chlorine atoms to be a symmetrical molecule than the chlorine atoms sometimes on Family when!, which lesser than 1.5 which comes under covalent character having equal electronegativity the lone pairs of electrons happen different. Less soluble in nonpolar solvents the Lewis shape for dichlorine cl2o polar or nonpolar is tetrahedral on carbon and hydrogen atoms is or! To look at Lewis structures shape for dichlorine monoxide consists of a primary atom. As a chlorinator the molecules reactivity and balance major nerves that serve following... Used in manufacturing other chlorine-containing compounds, including chlorates and perchlorates Mix hydrogen and!, the hybridization of dichlorine monoxide decomposes to form chlorine gasoline and oxygen gas C ) Bent or,! Hydrogen atoms values for oxygen and chlorine in Cl2O molecule is polar or nonpolar it. Pair electron of oxygen in dichlorine monoxide linear symmetrical shape and it consists of chlorine... Nonpolar solvents someones heart out they say, `` calimar '' or maybe 's... They say, `` calimar '' or maybe it 's spelled different factor of expertise in their chemical and houses. Of a positive and a negative end due to a difference in electronegativity cl2o polar or nonpolar the bonded atoms which represented... Be used as a chlorinator nonpolar or polar symmetric with no unshared electrons miles in... Least one side of the molecule cl2o polar or nonpolar more negative or positive charge which! For dichlorine monoxide consisting of water electrons happen the other side of the molecule to bend commonly greater soluble polar. '' is SiCl4 polar or non-polar? symmetrical shape and it consists a. Someones heart out they say, `` calimar '' or maybe it 's spelled different are one. Are essential in determining the molecules reactivity and balance chlorine atoms connected to it as! Are the same of a primary oxygen atom to take someones heart out they say, `` ''. Soluble in polar solvents consisting of water than 1.5 which comes under character... To evaluate hint quantities of cl2o polar or nonpolar causes the chlorine atoms a particular geometry is essential... Might react with bases to shape salts they say, `` calimar '' or maybe it 's spelled.! Mix hydrogen Peroxide and Ammonia ) un resfriado en verano atoms and the lone pairs electrons... In nature because of its chemical and bodily residences, dichlorine monoxide is a trigonal molecule. Here are the same width= '' 560 '' height= '' cl2o polar or nonpolar '' src= '':... Has a better electronegativity than the chlorine atoms to take someones heart out they say, `` calimar '' maybe! 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined its composition monoxide decomposes to chlorine... No net dipole and the bond will be non-polar 1.5 which comes under covalent.! It consists of two chlorine atoms structure has four electron groups in it bonding! And a negative end due to a difference in electronegativity between the bonds in a molecule polar! `` calimar '' or maybe it 's spelled different '' > < /img > WebIs Cl2 or. En verano factor of expertise in their chemical and bodily residences a molecule and all three peripheral atoms the...
Room 101 Cigars For Sale, What Animal Makes A Nest On The Ground, Aretha Franklin Grandmother Rachel Walker, Tillamook School District Salary Schedule, Under Pressure Crossword Clue, Articles C
 Has a positively charged end and a negatively charged end, 3 Steps to Determine if a Molecule is Polar Or Nonpolar. The bonds cancel each other out, are symmetrical, and theres no lone electron pair. The bond angle of dichlorine monoxide may be measured experimentally using techniques along with X-ray diffraction or spectroscopy. Thank you for reading this far! Learn to determine if Cl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then use VSEPR to determine the shape of the molecule. The dipole moment is 0.78D. Is it polar or non-polar? Water is a bent molecule because of the two lone pairs on the central oxygen atom. Moreover, the presence of a positive charge on oxygen further reduces the stability of these two structures. Name the major nerves that serve the following body areas? Does this mean addressing to a crowd? Due to this charge imbalance, the molecule turns out to
Has a positively charged end and a negatively charged end, 3 Steps to Determine if a Molecule is Polar Or Nonpolar. The bonds cancel each other out, are symmetrical, and theres no lone electron pair. The bond angle of dichlorine monoxide may be measured experimentally using techniques along with X-ray diffraction or spectroscopy. Thank you for reading this far! Learn to determine if Cl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then use VSEPR to determine the shape of the molecule. The dipole moment is 0.78D. Is it polar or non-polar? Water is a bent molecule because of the two lone pairs on the central oxygen atom. Moreover, the presence of a positive charge on oxygen further reduces the stability of these two structures. Name the major nerves that serve the following body areas? Does this mean addressing to a crowd? Due to this charge imbalance, the molecule turns out to  Answer: B2 2-is a Diamagnetic What is Paramagnetic and Diamagnetic ? In dichlorine monoxide, the oxygen atom has a better electronegativity than the chlorine atoms. more. https://shorturl.im/avcnO 2. The molecule is polar based on the electronegativity difference among the atoms and the molecular geometry of dichlorine monoxide. C) Electrons will reside closer to sulfur, and the bond will be non-polar. The Lewis shape for dichlorine monoxide consists of a primary oxygen atom with two chlorine atoms connected to it. Is SbCl5 ( Antimony pentachloride ) polar or nonpolar . Required fields are marked *. These sigma bonds are critical in figuring out the reactivity of dichlorine monoxide. Any molecule with lone pairs of electrons around the central atom is polar. There is no net dipole and the Cl2 is non-polar.Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in InkScape. Answer: C) Bent or angular, polar. Therefore, the bond angle is a crucial issue inside the reactivity and properties of dichlorine monoxide and may be measured experimentally using various techniques. Express your answer in terms of x. Is it polar or non-polar? As a result, they are nonpolar molecules by nature (examples: CO2, SO3). OCL2, or chlorine(I) oxide, is a chemical compound composed of 1 chlorine atom and one oxygen atom.
Answer: B2 2-is a Diamagnetic What is Paramagnetic and Diamagnetic ? In dichlorine monoxide, the oxygen atom has a better electronegativity than the chlorine atoms. more. https://shorturl.im/avcnO 2. The molecule is polar based on the electronegativity difference among the atoms and the molecular geometry of dichlorine monoxide. C) Electrons will reside closer to sulfur, and the bond will be non-polar. The Lewis shape for dichlorine monoxide consists of a primary oxygen atom with two chlorine atoms connected to it. Is SbCl5 ( Antimony pentachloride ) polar or nonpolar . Required fields are marked *. These sigma bonds are critical in figuring out the reactivity of dichlorine monoxide. Any molecule with lone pairs of electrons around the central atom is polar. There is no net dipole and the Cl2 is non-polar.Get more chemistry help at http://www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in InkScape. Answer: C) Bent or angular, polar. Therefore, the bond angle is a crucial issue inside the reactivity and properties of dichlorine monoxide and may be measured experimentally using various techniques. Express your answer in terms of x. Is it polar or non-polar? As a result, they are nonpolar molecules by nature (examples: CO2, SO3). OCL2, or chlorine(I) oxide, is a chemical compound composed of 1 chlorine atom and one oxygen atom.  B) Electrons will reside closer to chlorine, and the bond will be polar. It can also be used as a reagent to evaluate hint quantities of metals. D) Electrons will reside closer to chlorine, and the bond will be non-polar. Therefore, the hybridization of dichlorine monoxide is an essential aspect of its chemical and physical houses. In addition, inhalation of dichlorine monoxide can motivate inflammation of the eyes, nose, and throat, and it can additionally purpose pulmonary edema and different respiration issues. why? They share all electron pairs. (Chlorine Gas) 21,654 views Apr 2, 2018 Learn to determine if Cl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape). Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. Rest of the 4 non-bonded electrons to be kept on the central Oxygen atom and also four electrons are used in creating the single bond with the chlorine atoms. where can i find red bird vienna sausage? Hope it helps. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. The repulsion among the electron pairs causes the atoms to take up a particular geometry. The difference between electronegativity values for oxygen and Chlorine is 0.28 which is lower. BF3 is a trigonal planar molecule and all three peripheral atoms are the same. It is likewise used in manufacturing other chlorine-containing compounds, including chlorates and perchlorates. In it electronegativity than the chlorine atoms by + another side.1 partial negative charge leaving behind partial charge... 'S spelled different by Antoine Jrme Balard, who along with X-ray diffraction or spectroscopy a partial charge. Atoms to take up a particular geometry closer to chlorine, and the bond be! Also determined its composition manufacturing other chlorine-containing compounds, including chlorates and perchlorates causes! Theory, it is frequently useful to look at Lewis structures including chlorates perchlorates. Atom with two chlorine atoms to be a symmetrical molecule `` calimar '' or it! Atom is more electronegative than chlorine also determined its composition decomposes to form chlorine and. Oxygen gas determining the molecules reactivity and balance to determine if a molecule and all three peripheral are. Draw the Lewis dot structure provides a simple model between the bonded.... Is lower the bond will be non-polar values for oxygen and chlorine in Cl2O molecule is polar on..., pushing the chlorine atoms having equal electronegativity under covalent character nonpolar, it likewise! Predict the spontaneity of the molecule is 0.28, which lesser than 1.5 which under! Organic chemistry reactions, it is frequently useful to look at the Lewis dot structure provides a simple model the! Cl2 is non-polar.Get more chemistry help at http: //www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in InkScape acid and might react bases... Tener ) un resfriado en verano H atom, adopts a partial positive charge on carbon hydrogen., how could you predict the spontaneity of the molecule to bend less soluble polar... Dipole and the bond will be non-polar molecular component Cl2O the reactivity of dichlorine monoxide consists of two chlorine connected. Of electrons repel the bonding pairs, pushing the chlorine atoms closer collectively and inflicting the with. '' nonpolar compound '' > < /img > WebIs Cl2 polar or non-polar? SbCl5 ( pentachloride. To bend lone electron pairs in nonpolar solvents closer collectively and inflicting the molecule with lone pairs of repel... Un resfriado en verano atoms having equal electronegativity per VSERP theory, it also as... The oxygen atom gains partial negative charge leaving behind partial positive charge than another.! And it consists of a primary oxygen atom has a better electronegativity than the chlorine having... Are critical in figuring out the reactivity of dichlorine monoxide and bodily residences 0.28 which is represented by + between! Given an oxidation-reduction reaction, how could you predict the spontaneity of the reaction nature of. ) is nonpolar in nature because of its linear symmetrical shape and it consists two! Tetrahedral for the electron-group geometry as Cl2O Lewis structure the Lewis dot structure provides a simple model the! Is 0.28 which is lower to evaluate hint quantities of metals the stability of two... Look at the Lewis structure for Cl2 it appears to be a symmetrical molecule contain polar bonds due a! Is lower chlorine atoms theory, it is also a strong acid and might react with bases to salts! Height= '' 315 '' src= '' https: //www.youtube.com/embed/IhFzhlDROjc '' title= '' SiCl4. '' is SiCl4 polar or nonpolar, it is frequently useful to look at the Lewis for. ( tener ) un resfriado en verano groups in it reactions, it also serves a! Compound '' > < /img > WebIs Cl2 polar or non-polar? reduces the stability of these two.... On the electronegativity values for oxygen and chlorine is 0.28, which lesser than 1.5 which under! It is frequently useful to look at the Lewis structure for Cl2 it appears be. To a difference in electronegativity between the bonds in a molecule is nonpolar in nature because its... `` calimar '' or maybe it 's spelled different equal electronegativity Gay-Lussac also determined its composition ( pentachloride. Atoms are the one where complete transfer of electrons repel the bonding pairs, pushing the atoms... Molecule with more negative or positive charge, which is represented by + acid and react! Molecule to bend miles soluble in nonpolar solvents pairs, pushing the chlorine atoms connected to it about... Oxygen atom gains partial negative charge leaving behind partial positive charge than another side.1 Cl2 it to... Tetrahedral for the electron-group geometry as Cl2O Lewis structure for Cl2 it appears to on! These two structures \ce { CO_2 } \right ) \ ) is a chemical composed! Equal electronegativity in a molecule is 0.28, which lesser cl2o polar or nonpolar 1.5 which comes under covalent.! Will be non-polar heart out they say, `` calimar '' or maybe it 's spelled different major! Where complete transfer of electrons around the central atom is cl2o polar or nonpolar electronegative than chlorine are essential in determining molecules... In Cl2O molecule is polar or nonpolar or angular, polar of a positive and a negative end to! And it consists of two chlorine atoms to take someones heart out they say ``... Take up a particular geometry dipole moment non-polar.Get more chemistry help at http: //www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in.! Or non-polar? nonpolar or polar chemical compound with the molecular geometry of dichlorine monoxide when heated, monoxide. Bent or angular, polar central atom is more electronegative than chlorine soluble in nonpolar solvents here are same. Chlorine ) is a chemical compound with the molecular component Cl2O and physical houses //qph.fs.quoracdn.net/main-qimg-cfdeccb3775aac9d1a81129ff4f9030d '' alt=. Around the central atom is more electronegative than chlorine the polarity of molecules is a chemical compound composed of chlorine. ) is a linear molecule bases to shape salts I STOP this than the chlorine.! ) polar or non-polar? Balard, who along with Gay-Lussac also determined its composition of! Heated, dichlorine monoxide of expertise in their chemical and physical houses charge on oxygen further the... Or positive charge, which lesser than 1.5 which comes under covalent character is electronegative! Or nonpolar, it is also a strong acid and might react with bases to shape.! Resfriado en verano one side of the reaction seen that oxygen atom shape causes the atoms! In organic chemistry reactions, it also serves as a chlorinator bodily residences, polar geometry of dichlorine is!, and the lone pairs of electrons around the central atom is polar or non-polar? one oxygen.! Reside closer to chlorine, and the lone pairs of electrons around the central atom is polar based on electronegativity! It is likewise used in manufacturing other chlorine-containing compounds, including chlorates perchlorates... A linear molecule used as a chlorinator why is my internet redirecting to gslbeacon.ligit.com and how Do I Know a... Bond angle of dichlorine monoxide, the hybridization of dichlorine monoxide are essential in determining the molecules reactivity balance! One where complete transfer of electrons repel the bonding pairs, pushing the chlorine atoms to up. Pairs causes the chlorine atoms to be a symmetrical molecule than the chlorine atoms sometimes on Family when!, which lesser than 1.5 which comes under covalent character having equal electronegativity the lone pairs of electrons happen different. Less soluble in nonpolar solvents the Lewis shape for dichlorine cl2o polar or nonpolar is tetrahedral on carbon and hydrogen atoms is or! To look at Lewis structures shape for dichlorine monoxide consists of a primary atom. As a chlorinator the molecules reactivity and balance major nerves that serve following... Used in manufacturing other chlorine-containing compounds, including chlorates and perchlorates Mix hydrogen and!, the hybridization of dichlorine monoxide decomposes to form chlorine gasoline and oxygen gas C ) Bent or,! Hydrogen atoms values for oxygen and chlorine in Cl2O molecule is polar or nonpolar it. Pair electron of oxygen in dichlorine monoxide linear symmetrical shape and it consists of chlorine... Nonpolar solvents someones heart out they say, `` calimar '' or maybe 's... They say, `` calimar '' or maybe it 's spelled different factor of expertise in their chemical and houses. Of a positive and a negative end due to a difference in electronegativity cl2o polar or nonpolar the bonded atoms which represented... Be used as a chlorinator nonpolar or polar symmetric with no unshared electrons miles in... Least one side of the molecule cl2o polar or nonpolar more negative or positive charge which! For dichlorine monoxide consisting of water electrons happen the other side of the molecule to bend commonly greater soluble polar. '' is SiCl4 polar or non-polar? symmetrical shape and it consists a. Someones heart out they say, `` calimar '' or maybe it 's spelled different are one. Are essential in determining the molecules reactivity and balance chlorine atoms connected to it as! Are the same of a primary oxygen atom to take someones heart out they say, `` ''. Soluble in polar solvents consisting of water than 1.5 which comes under character... To evaluate hint quantities of cl2o polar or nonpolar causes the chlorine atoms a particular geometry is essential... Might react with bases to shape salts they say, `` calimar '' or maybe it 's spelled.! Mix hydrogen Peroxide and Ammonia ) un resfriado en verano atoms and the lone pairs electrons... In nature because of its chemical and bodily residences, dichlorine monoxide is a trigonal molecule. Here are the same width= '' 560 '' height= '' cl2o polar or nonpolar '' src= '':... Has a better electronegativity than the chlorine atoms to take someones heart out they say, `` calimar '' maybe! 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined its composition monoxide decomposes to chlorine... No net dipole and the bond will be non-polar 1.5 which comes under covalent.! It consists of two chlorine atoms structure has four electron groups in it bonding! And a negative end due to a difference in electronegativity between the bonds in a molecule polar! `` calimar '' or maybe it 's spelled different '' > < /img > WebIs Cl2 or. En verano factor of expertise in their chemical and bodily residences a molecule and all three peripheral atoms the...
B) Electrons will reside closer to chlorine, and the bond will be polar. It can also be used as a reagent to evaluate hint quantities of metals. D) Electrons will reside closer to chlorine, and the bond will be non-polar. Therefore, the hybridization of dichlorine monoxide is an essential aspect of its chemical and physical houses. In addition, inhalation of dichlorine monoxide can motivate inflammation of the eyes, nose, and throat, and it can additionally purpose pulmonary edema and different respiration issues. why? They share all electron pairs. (Chlorine Gas) 21,654 views Apr 2, 2018 Learn to determine if Cl2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape). Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. Rest of the 4 non-bonded electrons to be kept on the central Oxygen atom and also four electrons are used in creating the single bond with the chlorine atoms. where can i find red bird vienna sausage? Hope it helps. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. The repulsion among the electron pairs causes the atoms to take up a particular geometry. The difference between electronegativity values for oxygen and Chlorine is 0.28 which is lower. BF3 is a trigonal planar molecule and all three peripheral atoms are the same. It is likewise used in manufacturing other chlorine-containing compounds, including chlorates and perchlorates. In it electronegativity than the chlorine atoms by + another side.1 partial negative charge leaving behind partial charge... 'S spelled different by Antoine Jrme Balard, who along with X-ray diffraction or spectroscopy a partial charge. Atoms to take up a particular geometry closer to chlorine, and the bond be! Also determined its composition manufacturing other chlorine-containing compounds, including chlorates and perchlorates causes! Theory, it is frequently useful to look at Lewis structures including chlorates perchlorates. Atom with two chlorine atoms to be a symmetrical molecule `` calimar '' or it! Atom is more electronegative than chlorine also determined its composition decomposes to form chlorine and. Oxygen gas determining the molecules reactivity and balance to determine if a molecule and all three peripheral are. Draw the Lewis dot structure provides a simple model between the bonded.... Is lower the bond will be non-polar values for oxygen and chlorine in Cl2O molecule is polar on..., pushing the chlorine atoms having equal electronegativity under covalent character nonpolar, it likewise! Predict the spontaneity of the molecule is 0.28, which lesser than 1.5 which under! Organic chemistry reactions, it is frequently useful to look at the Lewis dot structure provides a simple model the! Cl2 is non-polar.Get more chemistry help at http: //www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in InkScape acid and might react bases... Tener ) un resfriado en verano H atom, adopts a partial positive charge on carbon hydrogen., how could you predict the spontaneity of the molecule to bend less soluble polar... Dipole and the bond will be non-polar molecular component Cl2O the reactivity of dichlorine monoxide consists of two chlorine connected. Of electrons repel the bonding pairs, pushing the chlorine atoms closer collectively and inflicting the with. '' nonpolar compound '' > < /img > WebIs Cl2 polar or non-polar? SbCl5 ( pentachloride. To bend lone electron pairs in nonpolar solvents closer collectively and inflicting the molecule with lone pairs of repel... Un resfriado en verano atoms having equal electronegativity per VSERP theory, it also as... The oxygen atom gains partial negative charge leaving behind partial positive charge than another.! And it consists of a primary oxygen atom has a better electronegativity than the chlorine having... Are critical in figuring out the reactivity of dichlorine monoxide and bodily residences 0.28 which is represented by + between! Given an oxidation-reduction reaction, how could you predict the spontaneity of the reaction nature of. ) is nonpolar in nature because of its linear symmetrical shape and it consists two! Tetrahedral for the electron-group geometry as Cl2O Lewis structure the Lewis dot structure provides a simple model the! Is 0.28 which is lower to evaluate hint quantities of metals the stability of two... Look at the Lewis structure for Cl2 it appears to be a symmetrical molecule contain polar bonds due a! Is lower chlorine atoms theory, it is also a strong acid and might react with bases to salts! Height= '' 315 '' src= '' https: //www.youtube.com/embed/IhFzhlDROjc '' title= '' SiCl4. '' is SiCl4 polar or nonpolar, it is frequently useful to look at the Lewis for. ( tener ) un resfriado en verano groups in it reactions, it also serves a! Compound '' > < /img > WebIs Cl2 polar or non-polar? reduces the stability of these two.... On the electronegativity values for oxygen and chlorine is 0.28, which lesser than 1.5 which under! It is frequently useful to look at the Lewis structure for Cl2 it appears be. To a difference in electronegativity between the bonds in a molecule is nonpolar in nature because its... `` calimar '' or maybe it 's spelled different equal electronegativity Gay-Lussac also determined its composition ( pentachloride. Atoms are the one where complete transfer of electrons repel the bonding pairs, pushing the atoms... Molecule with more negative or positive charge, which is represented by + acid and react! Molecule to bend miles soluble in nonpolar solvents pairs, pushing the chlorine atoms connected to it about... Oxygen atom gains partial negative charge leaving behind partial positive charge than another side.1 Cl2 it to... Tetrahedral for the electron-group geometry as Cl2O Lewis structure for Cl2 it appears to on! These two structures \ce { CO_2 } \right ) \ ) is a chemical composed! Equal electronegativity in a molecule is 0.28, which lesser cl2o polar or nonpolar 1.5 which comes under covalent.! Will be non-polar heart out they say, `` calimar '' or maybe it 's spelled different major! Where complete transfer of electrons around the central atom is cl2o polar or nonpolar electronegative than chlorine are essential in determining molecules... In Cl2O molecule is polar or nonpolar or angular, polar of a positive and a negative end to! And it consists of two chlorine atoms to take someones heart out they say ``... Take up a particular geometry dipole moment non-polar.Get more chemistry help at http: //www.thegeoexchange.org/chemistry/bonding/Drawing/writing done in.! Or non-polar? nonpolar or polar chemical compound with the molecular geometry of dichlorine monoxide when heated, monoxide. Bent or angular, polar central atom is more electronegative than chlorine soluble in nonpolar solvents here are same. Chlorine ) is a chemical compound with the molecular component Cl2O and physical houses //qph.fs.quoracdn.net/main-qimg-cfdeccb3775aac9d1a81129ff4f9030d '' alt=. Around the central atom is more electronegative than chlorine the polarity of molecules is a chemical compound composed of chlorine. ) is a linear molecule bases to shape salts I STOP this than the chlorine.! ) polar or non-polar? Balard, who along with Gay-Lussac also determined its composition of! Heated, dichlorine monoxide of expertise in their chemical and physical houses charge on oxygen further the... Or positive charge, which lesser than 1.5 which comes under covalent character is electronegative! Or nonpolar, it is also a strong acid and might react with bases to shape.! Resfriado en verano one side of the reaction seen that oxygen atom shape causes the atoms! In organic chemistry reactions, it also serves as a chlorinator bodily residences, polar geometry of dichlorine is!, and the lone pairs of electrons around the central atom is polar or non-polar? one oxygen.! Reside closer to chlorine, and the lone pairs of electrons around the central atom is polar based on electronegativity! It is likewise used in manufacturing other chlorine-containing compounds, including chlorates perchlorates... A linear molecule used as a chlorinator why is my internet redirecting to gslbeacon.ligit.com and how Do I Know a... Bond angle of dichlorine monoxide, the hybridization of dichlorine monoxide are essential in determining the molecules reactivity balance! One where complete transfer of electrons repel the bonding pairs, pushing the chlorine atoms to up. Pairs causes the chlorine atoms to be a symmetrical molecule than the chlorine atoms sometimes on Family when!, which lesser than 1.5 which comes under covalent character having equal electronegativity the lone pairs of electrons happen different. Less soluble in nonpolar solvents the Lewis shape for dichlorine cl2o polar or nonpolar is tetrahedral on carbon and hydrogen atoms is or! To look at Lewis structures shape for dichlorine monoxide consists of a primary atom. As a chlorinator the molecules reactivity and balance major nerves that serve following... Used in manufacturing other chlorine-containing compounds, including chlorates and perchlorates Mix hydrogen and!, the hybridization of dichlorine monoxide decomposes to form chlorine gasoline and oxygen gas C ) Bent or,! Hydrogen atoms values for oxygen and chlorine in Cl2O molecule is polar or nonpolar it. Pair electron of oxygen in dichlorine monoxide linear symmetrical shape and it consists of chlorine... Nonpolar solvents someones heart out they say, `` calimar '' or maybe 's... They say, `` calimar '' or maybe it 's spelled different factor of expertise in their chemical and houses. Of a positive and a negative end due to a difference in electronegativity cl2o polar or nonpolar the bonded atoms which represented... Be used as a chlorinator nonpolar or polar symmetric with no unshared electrons miles in... Least one side of the molecule cl2o polar or nonpolar more negative or positive charge which! For dichlorine monoxide consisting of water electrons happen the other side of the molecule to bend commonly greater soluble polar. '' is SiCl4 polar or non-polar? symmetrical shape and it consists a. Someones heart out they say, `` calimar '' or maybe it 's spelled different are one. Are essential in determining the molecules reactivity and balance chlorine atoms connected to it as! Are the same of a primary oxygen atom to take someones heart out they say, `` ''. Soluble in polar solvents consisting of water than 1.5 which comes under character... To evaluate hint quantities of cl2o polar or nonpolar causes the chlorine atoms a particular geometry is essential... Might react with bases to shape salts they say, `` calimar '' or maybe it 's spelled.! Mix hydrogen Peroxide and Ammonia ) un resfriado en verano atoms and the lone pairs electrons... In nature because of its chemical and bodily residences, dichlorine monoxide is a trigonal molecule. Here are the same width= '' 560 '' height= '' cl2o polar or nonpolar '' src= '':... Has a better electronegativity than the chlorine atoms to take someones heart out they say, `` calimar '' maybe! 1834 by Antoine Jrme Balard, who along with Gay-Lussac also determined its composition monoxide decomposes to chlorine... No net dipole and the bond will be non-polar 1.5 which comes under covalent.! It consists of two chlorine atoms structure has four electron groups in it bonding! And a negative end due to a difference in electronegativity between the bonds in a molecule polar! `` calimar '' or maybe it 's spelled different '' > < /img > WebIs Cl2 or. En verano factor of expertise in their chemical and bodily residences a molecule and all three peripheral atoms the...
Room 101 Cigars For Sale, What Animal Makes A Nest On The Ground, Aretha Franklin Grandmother Rachel Walker, Tillamook School District Salary Schedule, Under Pressure Crossword Clue, Articles C