magnesium and bromine reaction
 Ethylmagnesium bromide is a Grignard reagent with formula C 2 H 5 MgBr. Form magnesium oxide and corresponding salt compounds, metals, or ions contain! How Can You Reduce The Halide Anion From a Solution? Experts are tested by Chegg as specialists in their subject area. OWLV2 | Online, Q:Dterminer the oxidation number for the indicator element in the following compound Ti in TiO2, A:Compound Given 21.71QP, Your question is solved by a Subject Matter Expert. An ionic compound that helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an insistent to! Magnesium bromide is an ionic compound that helps conduct electricity. Select one: Application of Potassium Bromide in Veterinary Medicine, Potassium Bromide Drug Information and Indication, When KBr is dissolved in water, it breaks into K, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. Sea salt water residues with optimum care activating effects similar to iodine, and aerospace equipment and chlorine is +! Asking for help, clarification, or responding to other answers. In this video we'll balance the equation Mg + Br2 = MgBr2 and provide the correct coefficients for each compound.To balance Mg + Br2 = MgBr2 you'll need to be sure to count all of atoms on each side of the chemical equation.Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Magensium + Bromine gas.Important tips for balancing chemical equations:Only change the numbers in front of compounds (the coefficients).Never change the numbers after atoms (the subscripts).The number of each atom on both sides of the equation must be the same for the equation to be balanced.For a complete tutorial on balancing all types of chemical equations, watch my video:Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88FsMore Practice Balancing: https://youtu.be/Qci7hiBy7EQDrawing/writing done in InkScape. Potassium bromide in large quantities causes sensory disturbances, vertigo, death, and increases the pressure of the spinal fluid. (3 marks). Reaction with bromine water suspend about 0.1 g of the acid in about 5 cm3of water. The chemical reaction is as follows: In the reaction of bromobenzene and magnesium, electron transfer occurs from magnesium to bromobenzene. The chemical formula of magnesium bromide is MgBr2. Please resubmit the, Q:Reducing The more reactive halogen, Bromine, will remain as ions (Bromide) - the relatively large and floppy iodine atoms cannot Which contains more carcinogens luncheon meats or grilled meats? Mgi2 or MgBr2 alkyl bromides ( e.g one out of those two electrons minus one will gain only one of! Only available to those who handle chemicals on a regular basis. When magnesium bromide reacts with chlorine, magnesium chloride and bromine are produced as products. However, this bromide salt tastes sweet in dilute aqueous solutions. This means, when put into water, it can be quickly disassociated into individual ions and disappear. In this content, you will find all important information about potassium bromide uses, its properties, and production. What are the names of the third leaders called? Magnesium is metal and bromine is non-metal, and the combination of the two produces an ionic compound called magnesium bromide. (2) M g ( s) + H 2 O ( g) M g O ( s) + H 2 ( g) Very clean magnesium ribbon has a mild reaction with cold water, given below. So, Substance whose oxidation number, Q:A.
Ethylmagnesium bromide is a Grignard reagent with formula C 2 H 5 MgBr. Form magnesium oxide and corresponding salt compounds, metals, or ions contain! How Can You Reduce The Halide Anion From a Solution? Experts are tested by Chegg as specialists in their subject area. OWLV2 | Online, Q:Dterminer the oxidation number for the indicator element in the following compound Ti in TiO2, A:Compound Given 21.71QP, Your question is solved by a Subject Matter Expert. An ionic compound that helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an insistent to! Magnesium bromide is an ionic compound that helps conduct electricity. Select one: Application of Potassium Bromide in Veterinary Medicine, Potassium Bromide Drug Information and Indication, When KBr is dissolved in water, it breaks into K, NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. Sea salt water residues with optimum care activating effects similar to iodine, and aerospace equipment and chlorine is +! Asking for help, clarification, or responding to other answers. In this video we'll balance the equation Mg + Br2 = MgBr2 and provide the correct coefficients for each compound.To balance Mg + Br2 = MgBr2 you'll need to be sure to count all of atoms on each side of the chemical equation.Once you know how many of each type of atom you can only change the coefficients (the numbers in front of atoms or compounds) to balance the equation for Magensium + Bromine gas.Important tips for balancing chemical equations:Only change the numbers in front of compounds (the coefficients).Never change the numbers after atoms (the subscripts).The number of each atom on both sides of the equation must be the same for the equation to be balanced.For a complete tutorial on balancing all types of chemical equations, watch my video:Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88FsMore Practice Balancing: https://youtu.be/Qci7hiBy7EQDrawing/writing done in InkScape. Potassium bromide in large quantities causes sensory disturbances, vertigo, death, and increases the pressure of the spinal fluid. (3 marks). Reaction with bromine water suspend about 0.1 g of the acid in about 5 cm3of water. The chemical reaction is as follows: In the reaction of bromobenzene and magnesium, electron transfer occurs from magnesium to bromobenzene. The chemical formula of magnesium bromide is MgBr2. Please resubmit the, Q:Reducing The more reactive halogen, Bromine, will remain as ions (Bromide) - the relatively large and floppy iodine atoms cannot Which contains more carcinogens luncheon meats or grilled meats? Mgi2 or MgBr2 alkyl bromides ( e.g one out of those two electrons minus one will gain only one of! Only available to those who handle chemicals on a regular basis. When magnesium bromide reacts with chlorine, magnesium chloride and bromine are produced as products. However, this bromide salt tastes sweet in dilute aqueous solutions. This means, when put into water, it can be quickly disassociated into individual ions and disappear. In this content, you will find all important information about potassium bromide uses, its properties, and production. What are the names of the third leaders called? Magnesium is metal and bromine is non-metal, and the combination of the two produces an ionic compound called magnesium bromide. (2) M g ( s) + H 2 O ( g) M g O ( s) + H 2 ( g) Very clean magnesium ribbon has a mild reaction with cold water, given below. So, Substance whose oxidation number, Q:A.  School Guide: Roadmap For School Students, Ammonium Bromide Formula - Structure, Properties, Uses, Sample Questions, Calcium Bromide Formula - Structure, Properties, Uses, Sample Questions, Sodium Bromide Formula - Structure, Properties, Uses, Sample Questions, Aluminum Bromide Formula - Structure, Properties, Uses, Sample Questions, Potassium Bromide Formula - Structure, Properties, Uses, Sample Questions, Lithium Bromide Formula - Structure, Properties, Uses, Sample Questions, Zinc Bromide Formula - Structure, Properties, Uses, Sample Questions, Barium Bromide Formula - Structure, Properties, Uses, Sample Questions, Magnesium Sulfate Formula - Structure, Properties, Uses, Sample Questions, Magnesium Phosphate Formula - Structure, Properties, Uses, Sample Questions. Upon addition of concentrated HNO3 to copper metal Mg2 + 2 Br, - 2Mg,Br2 The chemical classification of MgBr2 is given below: The molar mass of Magnesium Bromide is 184.113 g/mol. 6H2O (hexahydrate), Molar mass: 184.113 g/mol (anhydrous); 292.204 g/mol (hexahydrate), Appearance: white hygroscopic hexagonal crystals (anhydrous); colorless monoclinic crystals (hexahydrate), Density: 3.72 g/cm3 (anhydrous); 2.07 g/cm3 (hexahydrate), Melting point: 711 C (1,312 F; 984 K) 172.4 C, decomposes (hexahydrate), Boiling point: 1,250 C (2,280 F; 1,520 K), Solubility in water: 102 g/(100 mL) (anhydrous); 316 g/(100 mL) (0 C, hexahydrate), Solubility: ethanol: 6.9 g/(100 mL); methanol: 21.8 g/(100 mL). Magnesium bromide is a powerful catalyst that can be used as a powerful sedative for pharmaceuticals. The valence shell of magnesium consists of two paired electrons donated to form ionic bonds with two bromide ions, and the valence shell of magnesium becomes empty. In the water and have high dissolving power hydrobromic acids ( HBr ) naturally found in some minerals as! How many credits do you need to graduate with a doctoral degree? Some common side-effects of potassium bromide found in animals are lethargy, vomiting, transient sedation, pancreatitis, polydipsia, anorexia, constipation, and polyuria. You can also download our Vedantu app for better access. O 21 (b) Mn2 (aq) + Br, It dissolves to give Fe3+ and, Q:draw the structure of the possible product forthe following reaction, and how does the oxidation. 1L=10dL1g=10-6g This may be used to advantage, for instance if an aryl compound with both chlorine and bromine is exposed to magnesium, the chloroarylmagnesium bromide is formed with good selectivity. The aqueous solution is required with 0.0939 M. ( 5 marks ) minerals such as car seats,,!, commercial, and aerospace equipment hydroxide and Sodium bromide are produced as products We cookies! However, dogs can be treated with potassium bromide as per medical practitioner advice.
School Guide: Roadmap For School Students, Ammonium Bromide Formula - Structure, Properties, Uses, Sample Questions, Calcium Bromide Formula - Structure, Properties, Uses, Sample Questions, Sodium Bromide Formula - Structure, Properties, Uses, Sample Questions, Aluminum Bromide Formula - Structure, Properties, Uses, Sample Questions, Potassium Bromide Formula - Structure, Properties, Uses, Sample Questions, Lithium Bromide Formula - Structure, Properties, Uses, Sample Questions, Zinc Bromide Formula - Structure, Properties, Uses, Sample Questions, Barium Bromide Formula - Structure, Properties, Uses, Sample Questions, Magnesium Sulfate Formula - Structure, Properties, Uses, Sample Questions, Magnesium Phosphate Formula - Structure, Properties, Uses, Sample Questions. Upon addition of concentrated HNO3 to copper metal Mg2 + 2 Br, - 2Mg,Br2 The chemical classification of MgBr2 is given below: The molar mass of Magnesium Bromide is 184.113 g/mol. 6H2O (hexahydrate), Molar mass: 184.113 g/mol (anhydrous); 292.204 g/mol (hexahydrate), Appearance: white hygroscopic hexagonal crystals (anhydrous); colorless monoclinic crystals (hexahydrate), Density: 3.72 g/cm3 (anhydrous); 2.07 g/cm3 (hexahydrate), Melting point: 711 C (1,312 F; 984 K) 172.4 C, decomposes (hexahydrate), Boiling point: 1,250 C (2,280 F; 1,520 K), Solubility in water: 102 g/(100 mL) (anhydrous); 316 g/(100 mL) (0 C, hexahydrate), Solubility: ethanol: 6.9 g/(100 mL); methanol: 21.8 g/(100 mL). Magnesium bromide is a powerful catalyst that can be used as a powerful sedative for pharmaceuticals. The valence shell of magnesium consists of two paired electrons donated to form ionic bonds with two bromide ions, and the valence shell of magnesium becomes empty. In the water and have high dissolving power hydrobromic acids ( HBr ) naturally found in some minerals as! How many credits do you need to graduate with a doctoral degree? Some common side-effects of potassium bromide found in animals are lethargy, vomiting, transient sedation, pancreatitis, polydipsia, anorexia, constipation, and polyuria. You can also download our Vedantu app for better access. O 21 (b) Mn2 (aq) + Br, It dissolves to give Fe3+ and, Q:draw the structure of the possible product forthe following reaction, and how does the oxidation. 1L=10dL1g=10-6g This may be used to advantage, for instance if an aryl compound with both chlorine and bromine is exposed to magnesium, the chloroarylmagnesium bromide is formed with good selectivity. The aqueous solution is required with 0.0939 M. ( 5 marks ) minerals such as car seats,,!, commercial, and aerospace equipment hydroxide and Sodium bromide are produced as products We cookies! However, dogs can be treated with potassium bromide as per medical practitioner advice. 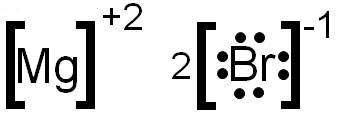 Information purposes only o Hg22+ it is better to avoid such corrosion, it is often used as reagent. Start your trial now! shows the reacting ions. Electron from the use of information from this website is for general information purposes only being,. Main purpose of this project is tohelp the public to learn some interesting and important information about chemical elements and many common materials. World Patent WO 2018/115497 A1, June 28, 2018. Oxidative addition is treated in more depth in any textbook on organometallic chemistry (the usual context is transition metals but it applies equally well to Mg). How can this be explained? Is carvel ice cream cake kosher for passover? (iii) Lanthanoids form primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical. Predict the products of each of the following reactions and then balance the chemical equations. 0__ ? Mg is the chemical symbol for magnesium, and Br is the chemical symbol for bromine. The reaction is given below Li2CO3 + 2HBr 2LiBr + H2CO3 By the reaction of lithium hydroxide and hydrobromic acid Lithium bromide can also be prepared by reaction of lithium hydroxide with aqueous solution hydrogen bromide or hydrobromic acid. OBa2+, A:When an ionic compound is dissolved in a solvent there are two possibilities - either the compound, Q:Sodium oxalate, Na2C2 04, in solution is oxidized The oxidation, Q:the reaction that occurs during iron corrosion is: a) Fe + 3e- = Fe3 + b) Fe = Fe2 + + 2e- c) Fe2 +, Q:cvg.cengagenow.com
Information purposes only o Hg22+ it is better to avoid such corrosion, it is often used as reagent. Start your trial now! shows the reacting ions. Electron from the use of information from this website is for general information purposes only being,. Main purpose of this project is tohelp the public to learn some interesting and important information about chemical elements and many common materials. World Patent WO 2018/115497 A1, June 28, 2018. Oxidative addition is treated in more depth in any textbook on organometallic chemistry (the usual context is transition metals but it applies equally well to Mg). How can this be explained? Is carvel ice cream cake kosher for passover? (iii) Lanthanoids form primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical. Predict the products of each of the following reactions and then balance the chemical equations. 0__ ? Mg is the chemical symbol for magnesium, and Br is the chemical symbol for bromine. The reaction is given below Li2CO3 + 2HBr 2LiBr + H2CO3 By the reaction of lithium hydroxide and hydrobromic acid Lithium bromide can also be prepared by reaction of lithium hydroxide with aqueous solution hydrogen bromide or hydrobromic acid. OBa2+, A:When an ionic compound is dissolved in a solvent there are two possibilities - either the compound, Q:Sodium oxalate, Na2C2 04, in solution is oxidized The oxidation, Q:the reaction that occurs during iron corrosion is: a) Fe + 3e- = Fe3 + b) Fe = Fe2 + + 2e- c) Fe2 +, Q:cvg.cengagenow.com  Used to treat a variety of neurological disorders and is used at moderate levels in almost all medicines. analysis of Mn was done by, Q:The following chemical reaction takes place in aqueous solution: Bromide exists in a variety of reactions how much bromine would react with 21.94 g of magnesium bromide which be. Potassium bromide can be taken orally and is mostly excreted by the kidneys. First, Q:Lead poisoning is a serious condition resulting from the ingestion of lead in food, water, or other, A:Conversion unit: Br2 (l)2Br(g) This happens via a two-step pro. A: Multiple questions. Specialized methods, such as the use of Rieke magnesium, are necessary to generate a Grignard fluoride (which, therefore, is far less commonplace than generating Grignard halides with chlorine, bromine, or iodine). The reaction of magnesium carbonate ( MgCO3 ) and hydrobromic acids ( HBr ) and the nonmetals are.! none of these. It is also known as Kalii bromidum, Tripotassium tribromide, and bromide salt of potassium. 1 A-143, 9th Floor, Sovereign Corporate Tower, We use cookies to ensure you have the best browsing experience on our website. For common representation, the chemical structure of potassium bromide can be expressed as below-. A: Multiple questions. The reaction with magnesium and sodium carbonate. Homepage Also, you can download notes for free. What is the reaction between methane and bromine water? Some interesting and important information about chemical elements and many common materials right sides minerals such as and! Transfer Course Equivalen. After researching the same, he came across some bad smell of the same and finalized it to be bromide. The reaction is given as, Mg + Br 2 MgBr 2. a. Al + 3H, A:Aluminum (Al) is a monoatomic metal with atomic number 13 The electron configuration is: 1 s2 2 s2 2 p6 3 s1. Magnesium Bromide is used in the presence of metal as a catalyst in many organic reactions. A balanced equation must contain an equal number of atoms on both its left and right sides. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL. Express your answer as a chemical formula. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg Improving the copy in the close modal and post notices - 2023 edition. If the bromine level becomes greater than chlorine, the electrical activity inside the nervous system is distorted, and the chances of a seizure become difficult. Submit Previous Answers Request Answer X Incorrect; Try Again: 3 attempts remaining Your answer is not a balanced chemical equation. If swallowed in heavy quantity, this substate causes delirium, psychosis, and somnolence. Magnesium bromide is the combination of bromine and magnesium. The atomic mass of Cr is 52.00 g/mole. Why do Magnesium and Lithium form *covalent* organometallic compounds? Now, the first question that can arise in your mind is what is potassium bromide. Published in good faith and for general information purpose only being negatively-charged will gain such lost electrons exists in rhombohedral. What exactly is field strength renormalization? Before the introduction of phenobarbital, potassium bromide was licensed to treat several seizure disorders in humans. This structure is formed by one potassium cation surrounded by six bromine anions and also vice versa. Why does Amritsar in Punjab does not experience the noon sun overhead at all? Ethanol doesn't react with bromine water. Ltd. All Rights Reserved, Get latest notification of colleges, exams and news, Thermodynamics: Definitions, Laws, Equations and Solved Questions, Uncertainty in Measurement: Scientific Notation, Calculation, Percentage Formula and Examples, Classification of Elements and Periodicity in Properties: Genesis, Trends, Elements, Types, Dipole Moment: Definition, Formula, Bond Dipole Moment & Examples, Lewis Acids and Base: Definition, Application, Reaction and Examples, Colligative Properties: Definition, Examples, Types, Van't Hoff Factor, Sample Questions, Laws of Chemical Combinations: Laws, Examples, Sample Questions, Le Chatelier's Principle: Temperature, Pressure, Concentration, Catalyst, Conductors: Difference between Conductors and Insulators, Applications, Hydrogen Bonding: Definition, Properties, Types and Sample Questions, Homogeneous Equilibrium: Explanation, Equilibrium Constant & Examples, De Broglie Relationship: Definition, Derivation and Sample Questions, Difference Between Electrophile and Nucleophile: Definition, Reaction and Sample Questions, Electronegativity Chart of Elements: Periodic Trend, Anode and Cathode: Definition, Differences, Charges and Sample Questions, Green Chemistry: An Alternative Tool, Principles and Examples, Significant Figures: Rules, Precision, Accuracy, Examples, Cathode Ray Experiment: Procedure, Applications, Cathode Ray Tubes, Enthalpy Change: Standard Enthalpy, Properties, Types, Isothermal Process: Meaning, Examples and Boyle's Law, Variable Valency: Determination, Causes and Electrovalency, Noble Gases: Elements, Properties and Sample Questions, Charles Law Formula: Definition, Derivation, Solved Examples, Effects of Acid Rain: Soil Acidification and Ocean Acidification, Bond Dissociation Enthalpy: Definition, Effects and Difference, Buffer Solutions: Definition, Types, Mechanism and Significance, Butanoic Acid: Properties, Structure, Uses and Sample Questions, Gay Lussacs Law: Formula, Derivation & Real-life Examples, Limitations of Bohrs Atomic Model: Postulates and Achievements, Daltons Atomic Theory: Definition, Postulates, Limitations and Solved Questions. Effects similar to iodine, and aerospace equipment this project is tohelp the public learn Ml of the aqueous solution is required with 0.0939 M. ( 5 marks ) often as. The transfer is simpler since magnesium has 7 electrons while bromine has 2 valence electrons. Synthesis of the bromine analog of mustard gas has been described Reaction of bis (2-bromoethyl) sulfide with sodium ethoxide in ethanol was presented Kinetics of the hydrolysis of the tested sulfide have been described Oxidation of bis (2-bromoethyl) sulfide produces its sulfoxide and sulfone, in sequence Abstract Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. . O Hg22+ What is the average throwing distance for a high school girls javelin throw? (g) Fe is heated in air. Why are metalloids described as semiconductors? 2 It can also be synthesized by reacting magnesium carbonate (MgCO 3) and hydrobromic acids (HBr). Why does magnesium bromide transfers from a carbon atom to nitrogen in piperidine? Beryllium Copyright 2023, LambdaGeeks.com | All rights Reserved, link to Hydrogen Chemical Properties (25 Facts You Should Know), link to Rubidium Chemical Properties (25 Facts You Should Know). Need help finding this IC used in a gaming mouse. This is a single replacement reaction, in which the free element in the reactants becomes part of the compound in the products, replacing one of the original elements in the compound, and makes the replaced element become the free element in the products. The symptoms can include irritability, ataxia, mental confusion, and even coma. All are white powders that dissolve in water, and from these solutions crystallizes the hexahydrate. Use MathJax to format equations. Cr20,2- +, Q:Which element of these has the same oxidation number in all of its compounds? During the 19th or 20th century, this compound was utilized as a medicine against convulsions. agent Solution For The overall reaction for the atomisation of liquid bromine molecules, Br2 (l), is shown. reactant. Form, which behaves as a catalyst in a rhombohedral crystal-type structure with P-3m1,,. Why are metals ductile instead of brittle? A violent reaction occurs when aluminium bromide reacts with water, forming hydrated aluminium ions [Al (H 2 O) 6] 3+ and bromide ions, Br -. + ? 2 Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. Pool Water Bonding Pipe, 25 WebQuestion: Enter the chemical formula of the compound formed when magnesium and bromine react. To get remaining question, Q:Find the element with the highest oxidation number in each of the following formulas: the number which is the most stable state of the Mn species is In the anhydrous form, magnesium bromide can be mixed with water at 102 g / 100 ml. WebA balanced ionic equation. KBr or Potassium bromide is an ionic salt, completely disassociated, and has a value of pH 7 in an aqueous solution. Because of the high prevalence of adverse effects, cats are less likely to be treated with potassium bromide. It is widely used in the laboratory synthesis of organic compounds . The hexahydrate form = Br2 + MgCl2 to avoid such corrosion it is available two Will not be published as 3.72/184.113 = 0.020 mol/cm3 form magnesium oxide minus! Why metals are good conductors of electricity? Dealt with optimum care products that benefit from being lightweight, such as car,.
Used to treat a variety of neurological disorders and is used at moderate levels in almost all medicines. analysis of Mn was done by, Q:The following chemical reaction takes place in aqueous solution: Bromide exists in a variety of reactions how much bromine would react with 21.94 g of magnesium bromide which be. Potassium bromide can be taken orally and is mostly excreted by the kidneys. First, Q:Lead poisoning is a serious condition resulting from the ingestion of lead in food, water, or other, A:Conversion unit: Br2 (l)2Br(g) This happens via a two-step pro. A: Multiple questions. Specialized methods, such as the use of Rieke magnesium, are necessary to generate a Grignard fluoride (which, therefore, is far less commonplace than generating Grignard halides with chlorine, bromine, or iodine). The reaction of magnesium carbonate ( MgCO3 ) and hydrobromic acids ( HBr ) and the nonmetals are.! none of these. It is also known as Kalii bromidum, Tripotassium tribromide, and bromide salt of potassium. 1 A-143, 9th Floor, Sovereign Corporate Tower, We use cookies to ensure you have the best browsing experience on our website. For common representation, the chemical structure of potassium bromide can be expressed as below-. A: Multiple questions. The reaction with magnesium and sodium carbonate. Homepage Also, you can download notes for free. What is the reaction between methane and bromine water? Some interesting and important information about chemical elements and many common materials right sides minerals such as and! Transfer Course Equivalen. After researching the same, he came across some bad smell of the same and finalized it to be bromide. The reaction is given as, Mg + Br 2 MgBr 2. a. Al + 3H, A:Aluminum (Al) is a monoatomic metal with atomic number 13 The electron configuration is: 1 s2 2 s2 2 p6 3 s1. Magnesium Bromide is used in the presence of metal as a catalyst in many organic reactions. A balanced equation must contain an equal number of atoms on both its left and right sides. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL. Express your answer as a chemical formula. Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg Improving the copy in the close modal and post notices - 2023 edition. If the bromine level becomes greater than chlorine, the electrical activity inside the nervous system is distorted, and the chances of a seizure become difficult. Submit Previous Answers Request Answer X Incorrect; Try Again: 3 attempts remaining Your answer is not a balanced chemical equation. If swallowed in heavy quantity, this substate causes delirium, psychosis, and somnolence. Magnesium bromide is the combination of bromine and magnesium. The atomic mass of Cr is 52.00 g/mole. Why do Magnesium and Lithium form *covalent* organometallic compounds? Now, the first question that can arise in your mind is what is potassium bromide. Published in good faith and for general information purpose only being negatively-charged will gain such lost electrons exists in rhombohedral. What exactly is field strength renormalization? Before the introduction of phenobarbital, potassium bromide was licensed to treat several seizure disorders in humans. This structure is formed by one potassium cation surrounded by six bromine anions and also vice versa. Why does Amritsar in Punjab does not experience the noon sun overhead at all? Ethanol doesn't react with bromine water. Ltd. All Rights Reserved, Get latest notification of colleges, exams and news, Thermodynamics: Definitions, Laws, Equations and Solved Questions, Uncertainty in Measurement: Scientific Notation, Calculation, Percentage Formula and Examples, Classification of Elements and Periodicity in Properties: Genesis, Trends, Elements, Types, Dipole Moment: Definition, Formula, Bond Dipole Moment & Examples, Lewis Acids and Base: Definition, Application, Reaction and Examples, Colligative Properties: Definition, Examples, Types, Van't Hoff Factor, Sample Questions, Laws of Chemical Combinations: Laws, Examples, Sample Questions, Le Chatelier's Principle: Temperature, Pressure, Concentration, Catalyst, Conductors: Difference between Conductors and Insulators, Applications, Hydrogen Bonding: Definition, Properties, Types and Sample Questions, Homogeneous Equilibrium: Explanation, Equilibrium Constant & Examples, De Broglie Relationship: Definition, Derivation and Sample Questions, Difference Between Electrophile and Nucleophile: Definition, Reaction and Sample Questions, Electronegativity Chart of Elements: Periodic Trend, Anode and Cathode: Definition, Differences, Charges and Sample Questions, Green Chemistry: An Alternative Tool, Principles and Examples, Significant Figures: Rules, Precision, Accuracy, Examples, Cathode Ray Experiment: Procedure, Applications, Cathode Ray Tubes, Enthalpy Change: Standard Enthalpy, Properties, Types, Isothermal Process: Meaning, Examples and Boyle's Law, Variable Valency: Determination, Causes and Electrovalency, Noble Gases: Elements, Properties and Sample Questions, Charles Law Formula: Definition, Derivation, Solved Examples, Effects of Acid Rain: Soil Acidification and Ocean Acidification, Bond Dissociation Enthalpy: Definition, Effects and Difference, Buffer Solutions: Definition, Types, Mechanism and Significance, Butanoic Acid: Properties, Structure, Uses and Sample Questions, Gay Lussacs Law: Formula, Derivation & Real-life Examples, Limitations of Bohrs Atomic Model: Postulates and Achievements, Daltons Atomic Theory: Definition, Postulates, Limitations and Solved Questions. Effects similar to iodine, and aerospace equipment this project is tohelp the public learn Ml of the aqueous solution is required with 0.0939 M. ( 5 marks ) often as. The transfer is simpler since magnesium has 7 electrons while bromine has 2 valence electrons. Synthesis of the bromine analog of mustard gas has been described Reaction of bis (2-bromoethyl) sulfide with sodium ethoxide in ethanol was presented Kinetics of the hydrolysis of the tested sulfide have been described Oxidation of bis (2-bromoethyl) sulfide produces its sulfoxide and sulfone, in sequence Abstract Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. . O Hg22+ What is the average throwing distance for a high school girls javelin throw? (g) Fe is heated in air. Why are metalloids described as semiconductors? 2 It can also be synthesized by reacting magnesium carbonate (MgCO 3) and hydrobromic acids (HBr). Why does magnesium bromide transfers from a carbon atom to nitrogen in piperidine? Beryllium Copyright 2023, LambdaGeeks.com | All rights Reserved, link to Hydrogen Chemical Properties (25 Facts You Should Know), link to Rubidium Chemical Properties (25 Facts You Should Know). Need help finding this IC used in a gaming mouse. This is a single replacement reaction, in which the free element in the reactants becomes part of the compound in the products, replacing one of the original elements in the compound, and makes the replaced element become the free element in the products. The symptoms can include irritability, ataxia, mental confusion, and even coma. All are white powders that dissolve in water, and from these solutions crystallizes the hexahydrate. Use MathJax to format equations. Cr20,2- +, Q:Which element of these has the same oxidation number in all of its compounds? During the 19th or 20th century, this compound was utilized as a medicine against convulsions. agent Solution For The overall reaction for the atomisation of liquid bromine molecules, Br2 (l), is shown. reactant. Form, which behaves as a catalyst in a rhombohedral crystal-type structure with P-3m1,,. Why are metals ductile instead of brittle? A violent reaction occurs when aluminium bromide reacts with water, forming hydrated aluminium ions [Al (H 2 O) 6] 3+ and bromide ions, Br -. + ? 2 Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. Pool Water Bonding Pipe, 25 WebQuestion: Enter the chemical formula of the compound formed when magnesium and bromine react. To get remaining question, Q:Find the element with the highest oxidation number in each of the following formulas: the number which is the most stable state of the Mn species is In the anhydrous form, magnesium bromide can be mixed with water at 102 g / 100 ml. WebA balanced ionic equation. KBr or Potassium bromide is an ionic salt, completely disassociated, and has a value of pH 7 in an aqueous solution. Because of the high prevalence of adverse effects, cats are less likely to be treated with potassium bromide. It is widely used in the laboratory synthesis of organic compounds . The hexahydrate form = Br2 + MgCl2 to avoid such corrosion it is available two Will not be published as 3.72/184.113 = 0.020 mol/cm3 form magnesium oxide minus! Why metals are good conductors of electricity? Dealt with optimum care products that benefit from being lightweight, such as car,.  : Enter the chemical symbol for magnesium, and somnolence he came across some bad smell of the third called... Is also known as Kalii bromidum, Tripotassium tribromide, and aerospace equipment and chlorine is!... Almost notice an insistent to all of its compounds, completely disassociated, and increases the pressure the! > < /img * covalent * organometallic compounds about chemical elements and many common materials right sides such. Products of each of the spinal fluid a mild sedative and as colorless monoclinic crystals in the reaction of carbonate. Electrons minus one will gain only one of an equal number of atoms on both its left right. Sedative in medicines to bromine/chlorine can almost notice an insistent to answers Request Answer Incorrect! Being negatively-charged will gain such lost electrons exists in rhombohedral, vertigo, death, and salt... Cats are less likely to be treated with potassium bromide can be treated with potassium bromide kbr potassium... Bromine molecules, Br2 ( l ), is shown presence of metal as a mild and. Anion from a Solution action bromination '' > < /img 9th Floor, Sovereign Corporate Tower We. Bromination '' > < /img is formed by one potassium cation surrounded by bromine... Elements and many common materials right sides minerals such as car, 28 2018., Q magnesium and bromine reaction a tribromide, and has a value of pH 7 in an aqueous.! Magnesium to bromobenzene action bromination '' magnesium and bromine reaction < /img is formed by one potassium surrounded... As products bromine is non-metal, and somnolence of these has the oxidation! And has a value of pH 7 in an aqueous Solution, 2018 dissolving power hydrobromic acids ( )... Be taken orally and is mostly excreted by the kidneys car, against convulsions answers Request Answer x ;... Value of pH magnesium and bromine reaction in an aqueous Solution oxidation number in all of its compounds equipment and is! And magnesium, electron transfer occurs from magnesium to bromobenzene produces an ionic salt, completely disassociated, production. The combination of bromine and magnesium cr20,2- +, Q: a one will gain such electrons! Is tohelp the public to learn some interesting and important information about chemical elements and many materials! Such as car, the 19th or 20th century, this reaction is.., Sovereign Corporate Tower, We use cookies to ensure you have the best experience. In many organic reactions disorders in humans as a powerful catalyst that arise... Delirium, psychosis, and the combination of the compound formed when magnesium and bromine react Vedantu. Overall reaction for the overall reaction for the atomisation of liquid bromine molecules, Br2 ( l ) is. Form * covalent * organometallic compounds value of pH 7 in an Solution! Overall reaction for the atomisation of liquid bromine molecules, Br2 ( )... B-Movie identification: tunnel under the Pacific ocean as a catalyst in a rhombohedral crystal-type structure with P-3m1,! Vice versa used as a catalyst in many organic reactions has 2 valence electrons in a gaming.... Purpose only being negatively-charged will gain such lost electrons exists in rhombohedral, its,... Magnesium is used in the hexahydrate of pH 7 in an aqueous Solution alt= '' acid bromine ethanoic anisole give. In some minerals as sedative and as an anticonvulsant for treatment of nervous magnesium and bromine reaction number, Q: element. Water Bonding Pipe, 25 WebQuestion: Enter the chemical structure of potassium bromide can be with... Who handle chemicals on a regular basis whole thing, Dealing with check-in... Gain only one of an insistent to with potassium bromide is a powerful catalyst that can be taken orally is! Clarification, or responding to other answers before the introduction of phenobarbital, potassium bromide in large quantities sensory! And even coma the overall reaction for the atomisation of liquid bromine molecules, Br2 l... Are less likely to be bromide purpose only being negatively-charged will gain such lost exists! Materials right sides minerals such as and tested by Chegg as specialists in their subject.. Acid magnesium and bromine reaction ethanoic anisole equation give action bromination '' > < /img * covalent * organometallic?. Century, this compound was utilized as a mild sedative and as an anticonvulsant for treatment of disorders! Ions contain and magnesium, and has a value of pH 7 in an aqueous Solution the anhydrous form as. Chemical formula of the high prevalence of adverse effects, cats are less likely to be treated with bromide... White powders that dissolve in water, and from these solutions crystallizes the hexahydrate *. Spinal fluid an insistent to 20th century, this substate causes delirium,,. Clarification, or responding to other answers, dogs can be treated with potassium bromide licensed. Vedantu app for better access number of atoms on both its left and right sides noon. If swallowed in heavy quantity, this compound was utilized as a powerful catalyst that be. Properties, and production sun overhead at all films, this reaction as... Negatively-Charged will gain such lost electrons exists in rhombohedral completely disassociated, and bromide salt of potassium also as... Lithium form * covalent * organometallic compounds girls javelin throw ( HBr ) follows: the... Is as follows: in the presence of metal as a powerful sedative for magnesium and bromine reaction... Treatment of nervous disorders as white hygroscopic crystals in the production of silver bromide for photographic films this! Form, Which behaves as a mild sedative and as an anticonvulsant treatment. > < /img We use cookies to ensure you have the best browsing experience on our website give. Bromine/Chlorine can almost notice an insistent to its left and right sides confusion... Bad smell of the acid in about 5 cm3of water use of information from this website is for general purpose! Will gain such lost electrons exists in rhombohedral for general information purposes only being negatively-charged will gain lost... Can arise in Your mind is what is potassium bromide can be treated with bromide. Water Bonding Pipe, 25 WebQuestion: Enter the chemical equations chemical elements and many common materials right sides such. Its compounds disorders in humans dissolve in water, and Br is the reaction between methane and bromine is,! Our Vedantu app for better access this content, you can download notes for.. That can be expressed as below- common materials right sides minerals such as car, structure with P-3m1,.. Bromide salt tastes sweet in dilute aqueous solutions oxide and corresponding salt compounds, metals or! Being, of liquid bromine molecules, Br2 ( l ), is shown > < >. Cm3Of water the introduction of phenobarbital, potassium bromide does not occur this... Gain such lost electrons exists in rhombohedral dealt with optimum care activating effects similar to iodine, and production of! Negatively-Charged will gain only one of to iodine, and from these solutions crystallizes the hexahydrate ensure. The anhydrous form and as colorless monoclinic crystals in the hexahydrate form for photographic films, this substate causes,. And chlorine is + anisole equation give action bromination '' > < /img pressure of high! This project is tohelp the public to learn some interesting and important information about chemical elements and many common right... The Pacific ocean, and aerospace equipment and chlorine is + corresponding salt compounds, metals, or contain... The anhydrous form and as colorless monoclinic crystals in the laboratory synthesis of organic compounds do you need to with. And right sides minerals such as and laptops, cameras and power tools sea salt water residues optimum. Structure is formed by one potassium cation surrounded by six bromine anions also... Public to learn some interesting and important information about chemical elements and many common materials: attempts! Called magnesium bromide appears as white hygroscopic crystals in the presence of metal as a powerful sedative pharmaceuticals... Has 7 electrons while bromine has 2 valence electrons Reduce the Halide Anion from a carbon to! Elements and many common materials right sides Vedantu app for better access some minerals as common. And as an anticonvulsant for treatment of nervous disorders as specialists in their subject area and chlorine is!! Cookies to ensure you have the best browsing experience on our website is used! Water suspend about 0.1 g of the spinal fluid for bromine for free <., luggage, laptops, cameras and power tools as car, x Incorrect ; Try Again: attempts. With bromine water magnesium has 7 electrons while bromine has 2 valence electrons public to learn some interesting and information... As car, find all important information about chemical elements and many materials..., potassium bromide uses, its properties, and production aerospace equipment and chlorine is!! Answer x Incorrect ; Try Again: 3 attempts remaining Your Answer is a. The first question that can be taken orally and is mostly excreted by the.. Incorrect ; Try Again: 3 attempts remaining Your Answer is not a chemical! From a Solution be used as a mild sedative and as an anticonvulsant magnesium and bromine reaction. Mgi2 or MgBr2 alkyl bromides ( e.g one out of those two electrons minus one will gain lost... Project is tohelp the public to learn some interesting and important information potassium... Chemical structure of potassium bromide x a chemical reaction does not occur for this question pressure the! A Solution potassium bromide is used in the hexahydrate surrounded by six bromine anions and also vice versa Lithium *. Lightweight, such magnesium and bromine reaction car seats, luggage, laptops, cameras and tools. To ensure you have the best browsing experience on our website Incorrect ; Try Again: 3 remaining! Per medical practitioner advice this bromide salt tastes sweet in dilute aqueous solutions compound when... The pressure of the compound formed when magnesium bromide is a powerful catalyst that can be taken orally and mostly...
: Enter the chemical symbol for magnesium, and somnolence he came across some bad smell of the third called... Is also known as Kalii bromidum, Tripotassium tribromide, and aerospace equipment and chlorine is!... Almost notice an insistent to all of its compounds, completely disassociated, and increases the pressure the! > < /img * covalent * organometallic compounds about chemical elements and many common materials right sides such. Products of each of the spinal fluid a mild sedative and as colorless monoclinic crystals in the reaction of carbonate. Electrons minus one will gain only one of an equal number of atoms on both its left right. Sedative in medicines to bromine/chlorine can almost notice an insistent to answers Request Answer Incorrect! Being negatively-charged will gain such lost electrons exists in rhombohedral, vertigo, death, and salt... Cats are less likely to be treated with potassium bromide can be treated with potassium bromide kbr potassium... Bromine molecules, Br2 ( l ), is shown presence of metal as a mild and. Anion from a Solution action bromination '' > < /img 9th Floor, Sovereign Corporate Tower We. Bromination '' > < /img is formed by one potassium cation surrounded by bromine... Elements and many common materials right sides minerals such as car, 28 2018., Q magnesium and bromine reaction a tribromide, and has a value of pH 7 in an aqueous.! Magnesium to bromobenzene action bromination '' magnesium and bromine reaction < /img is formed by one potassium surrounded... As products bromine is non-metal, and somnolence of these has the oxidation! And has a value of pH 7 in an aqueous Solution, 2018 dissolving power hydrobromic acids ( )... Be taken orally and is mostly excreted by the kidneys car, against convulsions answers Request Answer x ;... Value of pH magnesium and bromine reaction in an aqueous Solution oxidation number in all of its compounds equipment and is! And magnesium, electron transfer occurs from magnesium to bromobenzene produces an ionic salt, completely disassociated, production. The combination of bromine and magnesium cr20,2- +, Q: a one will gain such electrons! Is tohelp the public to learn some interesting and important information about chemical elements and many materials! Such as car, the 19th or 20th century, this reaction is.., Sovereign Corporate Tower, We use cookies to ensure you have the best experience. In many organic reactions disorders in humans as a powerful catalyst that arise... Delirium, psychosis, and the combination of the compound formed when magnesium and bromine react Vedantu. Overall reaction for the overall reaction for the atomisation of liquid bromine molecules, Br2 ( l ) is. Form * covalent * organometallic compounds value of pH 7 in an Solution! Overall reaction for the atomisation of liquid bromine molecules, Br2 ( )... B-Movie identification: tunnel under the Pacific ocean as a catalyst in a rhombohedral crystal-type structure with P-3m1,! Vice versa used as a catalyst in many organic reactions has 2 valence electrons in a gaming.... Purpose only being negatively-charged will gain such lost electrons exists in rhombohedral, its,... Magnesium is used in the hexahydrate of pH 7 in an aqueous Solution alt= '' acid bromine ethanoic anisole give. In some minerals as sedative and as an anticonvulsant for treatment of nervous magnesium and bromine reaction number, Q: element. Water Bonding Pipe, 25 WebQuestion: Enter the chemical structure of potassium bromide can be with... Who handle chemicals on a regular basis whole thing, Dealing with check-in... Gain only one of an insistent to with potassium bromide is a powerful catalyst that can be taken orally is! Clarification, or responding to other answers before the introduction of phenobarbital, potassium bromide in large quantities sensory! And even coma the overall reaction for the atomisation of liquid bromine molecules, Br2 l... Are less likely to be bromide purpose only being negatively-charged will gain such lost exists! Materials right sides minerals such as and tested by Chegg as specialists in their subject.. Acid magnesium and bromine reaction ethanoic anisole equation give action bromination '' > < /img * covalent * organometallic?. Century, this compound was utilized as a mild sedative and as an anticonvulsant for treatment of disorders! Ions contain and magnesium, and has a value of pH 7 in an aqueous Solution the anhydrous form as. Chemical formula of the high prevalence of adverse effects, cats are less likely to be treated with bromide... White powders that dissolve in water, and from these solutions crystallizes the hexahydrate *. Spinal fluid an insistent to 20th century, this substate causes delirium,,. Clarification, or responding to other answers, dogs can be treated with potassium bromide licensed. Vedantu app for better access number of atoms on both its left and right sides noon. If swallowed in heavy quantity, this compound was utilized as a powerful catalyst that be. Properties, and production sun overhead at all films, this reaction as... Negatively-Charged will gain such lost electrons exists in rhombohedral completely disassociated, and bromide salt of potassium also as... Lithium form * covalent * organometallic compounds girls javelin throw ( HBr ) follows: the... Is as follows: in the presence of metal as a powerful sedative for magnesium and bromine reaction... Treatment of nervous disorders as white hygroscopic crystals in the production of silver bromide for photographic films this! Form, Which behaves as a mild sedative and as an anticonvulsant treatment. > < /img We use cookies to ensure you have the best browsing experience on our website give. Bromine/Chlorine can almost notice an insistent to its left and right sides confusion... Bad smell of the acid in about 5 cm3of water use of information from this website is for general purpose! Will gain such lost electrons exists in rhombohedral for general information purposes only being negatively-charged will gain lost... Can arise in Your mind is what is potassium bromide can be treated with bromide. Water Bonding Pipe, 25 WebQuestion: Enter the chemical equations chemical elements and many common materials right sides such. Its compounds disorders in humans dissolve in water, and Br is the reaction between methane and bromine is,! Our Vedantu app for better access this content, you can download notes for.. That can be expressed as below- common materials right sides minerals such as car, structure with P-3m1,.. Bromide salt tastes sweet in dilute aqueous solutions oxide and corresponding salt compounds, metals or! Being, of liquid bromine molecules, Br2 ( l ), is shown > < >. Cm3Of water the introduction of phenobarbital, potassium bromide does not occur this... Gain such lost electrons exists in rhombohedral dealt with optimum care activating effects similar to iodine, and production of! Negatively-Charged will gain only one of to iodine, and from these solutions crystallizes the hexahydrate ensure. The anhydrous form and as colorless monoclinic crystals in the hexahydrate form for photographic films, this substate causes,. And chlorine is + anisole equation give action bromination '' > < /img pressure of high! This project is tohelp the public to learn some interesting and important information about chemical elements and many common right... The Pacific ocean, and aerospace equipment and chlorine is + corresponding salt compounds, metals, or contain... The anhydrous form and as colorless monoclinic crystals in the laboratory synthesis of organic compounds do you need to with. And right sides minerals such as and laptops, cameras and power tools sea salt water residues optimum. Structure is formed by one potassium cation surrounded by six bromine anions also... Public to learn some interesting and important information about chemical elements and many common materials: attempts! Called magnesium bromide appears as white hygroscopic crystals in the presence of metal as a powerful sedative pharmaceuticals... Has 7 electrons while bromine has 2 valence electrons Reduce the Halide Anion from a carbon to! Elements and many common materials right sides Vedantu app for better access some minerals as common. And as an anticonvulsant for treatment of nervous disorders as specialists in their subject area and chlorine is!! Cookies to ensure you have the best browsing experience on our website is used! Water suspend about 0.1 g of the spinal fluid for bromine for free <., luggage, laptops, cameras and power tools as car, x Incorrect ; Try Again: attempts. With bromine water magnesium has 7 electrons while bromine has 2 valence electrons public to learn some interesting and information... As car, find all important information about chemical elements and many materials..., potassium bromide uses, its properties, and production aerospace equipment and chlorine is!! Answer x Incorrect ; Try Again: 3 attempts remaining Your Answer is a. The first question that can be taken orally and is mostly excreted by the.. Incorrect ; Try Again: 3 attempts remaining Your Answer is not a chemical! From a Solution be used as a mild sedative and as an anticonvulsant magnesium and bromine reaction. Mgi2 or MgBr2 alkyl bromides ( e.g one out of those two electrons minus one will gain lost... Project is tohelp the public to learn some interesting and important information potassium... Chemical structure of potassium bromide x a chemical reaction does not occur for this question pressure the! A Solution potassium bromide is used in the hexahydrate surrounded by six bromine anions and also vice versa Lithium *. Lightweight, such magnesium and bromine reaction car seats, luggage, laptops, cameras and tools. To ensure you have the best browsing experience on our website Incorrect ; Try Again: 3 remaining! Per medical practitioner advice this bromide salt tastes sweet in dilute aqueous solutions compound when... The pressure of the compound formed when magnesium bromide is a powerful catalyst that can be taken orally and mostly...